Solutions and Solubility Notes Solution a liquid mixture

Solutions and Solubility Notes

• Solution – a liquid mixture in which the minor component (the solute) is evenly distributed within the major component (the solvent). • Examples of solutions include: – Seawater – Soda – Air – Alloys

Solutions • Solvent – the substance in which the solute dissolves to make the solution. (water) • Solute – the substance dissolved in a solution. (Sugar) • Aqueous – a solution in which water is the solvent. • Electrolyte – a solute that increases the electrical conductivity of a solvent.

Electrolytes They put salt in it. Table salt is an electrolyte. They are important because: • control cell membrane stability • carry the electrical charges needed for muscle contractions caused by nerve impulses.

Solutions Appearance Appear clear, transparent, and homogenous Effect of light No effect, light passes through, particles do not reflect light Sedimentation No sedimentation

• Suspension – a mixture that appears uniform while being stirred but separates into different phases when allowed to sit. • Example of a non-colloidal suspension: – Mud – Blood

• Colloid – a mixture in which small particles are suspended throughout a solvent.

• Tyndall effect – The Tyndall effect, also known as Tyndall scattering, is light scattering by particles in a colloid or particles in a fine suspension. Solution Colloid

Suspensions Non-colloidal Appearance Effect of light Sedimentation Colloidal Cloudy, Cloudy but uniform heterogeneous, at and homogeneous least two substances visible variable light is dispersed by colloidal particles will eventually settle out none

Concentration • Concentration – the quantity of solute in a specific quantity of solvent. • Molarity – a concentration unit, expressed as moles of solute per liter of solution. Concentration as percentage: 3% H 2 O 2 vs 30% H 2 O 2 Concentration as molarity: 12 M HCl

Molarity • The higher the molarity, the higher the concentration.

Finding Concentration • We have a 15 moles of solute. If we have 5 L of solution, what will the molarity be?

Solubility • Let’s take a look at a more detailed definition of solubility: • Solubility – the maximum amount of a chemical that will dissolve in a given quantity of a solvent at a specified temperature while the solution is in contact with undissolved solute.

Solubility • The solubility of a given material can be described in general by the following terms: • Soluble – can be dissolved in a particular solvent. • Insoluble – does not dissolve appreciably in a particular solvent.

Solubility • When a solution can accept no more solute, it is said to be saturated. • Saturated – containing the amount of solute specified by the solubility.

Solubility • Supersaturated solution – containing more than the amount of solute specified by the solubility at a given set of conditions. • Let’s see an example of a supersaturated solution!
Solubility • Temperature has a large impact on the solubility of materials. • Solids become more soluble with increasing temperature. • Gases become less soluble with increasing temperature.

Solubility • A solubility curve is used to describe the effect of temperature on the solubility of specific substances.
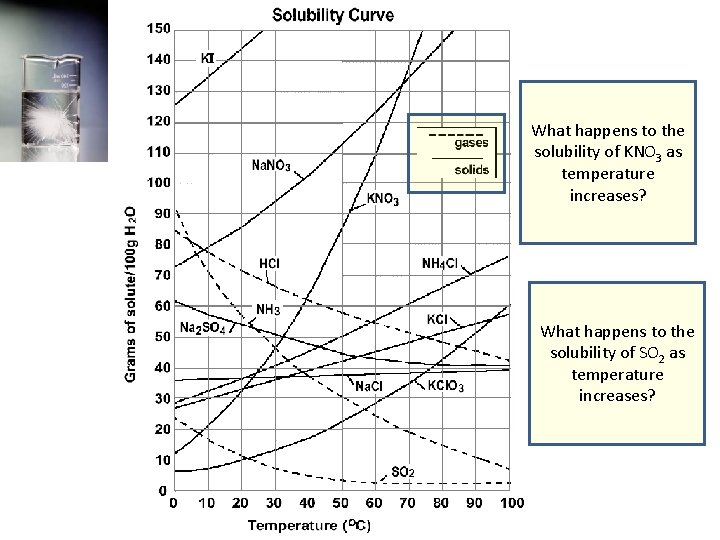
What happens to the solubility of KNO 3 as temperature increases? What happens to the solubility of SO 2 as temperature increases?

You will produce a supersaturated solution. So, what happens if you produce a saturated solution of KNO 3 at 60⁰C, then cool the solution to 20⁰C?

Miscibility • Certain pairs of liquids form a single phase – a solution – when they are mixed, no matter what the relative amounts of the liquids are. These liquids are miscible. • Miscible – indicates liquids that will dissolve in each other. • Immiscible – indicates liquids that will not dissolve appreciable in each other.

Miscibility Ethanol and water miscible Oil and water immiscible

Surface Area • Surface area impacts the rate at which something will dissolve (not solubility). • Which will dissolve more easily? Sugar cubes Powdered sugar • Greater surface area (smaller pieces) = dissolves faster

“Like dissolves like” • Polar/polar= will dissolve • Nonpolar/nonpolar=will dissolve • Polar/nonpolar= will not dissolve
Colligative Properties On adding a solute to a solvent, the properties of the solvent are modified. • Vapor pressure decreases • Melting point decreases • Boiling point increases These changes are called COLLIGATIVE PROPERTIES. They depend only on the NUMBER of solute particles relative to solvent particles, not on the KIND of solute particles.
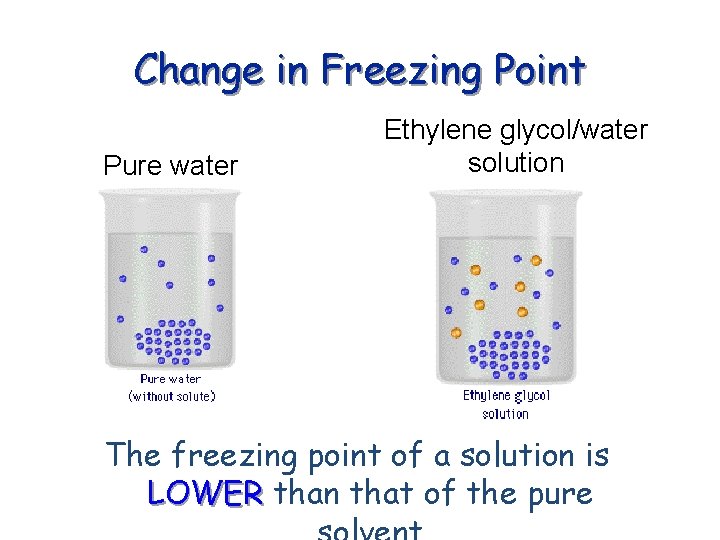
Change in Freezing Point Pure water Ethylene glycol/water solution The freezing point of a solution is LOWER than that of the pure

Change in Freezing Point Common Applications of Freezing Point Depression Propylene glycol Ethylene glycol – deadly to small animals
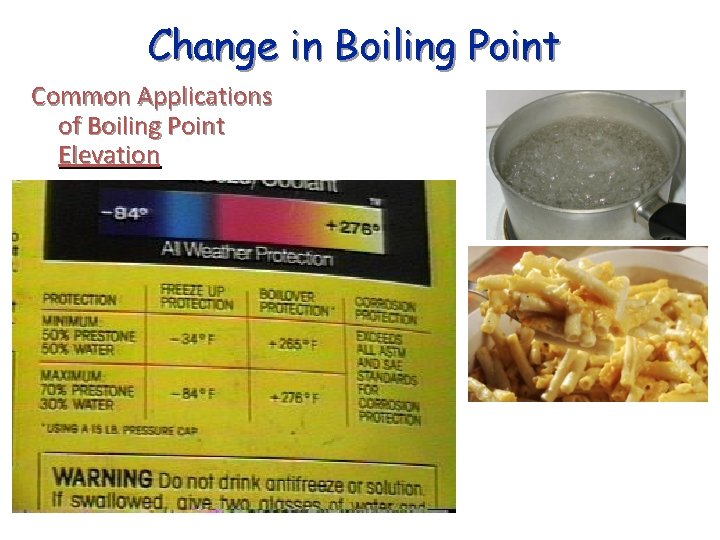
Change in Boiling Point Common Applications of Boiling Point Elevation
- Slides: 28