Solutions and Solubility Concentration of Solutions The concentration

Solutions and Solubility

Concentration of Solutions ● ● ● The concentration of a solution describes the relative amounts of the solute and solvent. Concentrated solution contain large amounts of solute. Dilute solutions contain little solute.

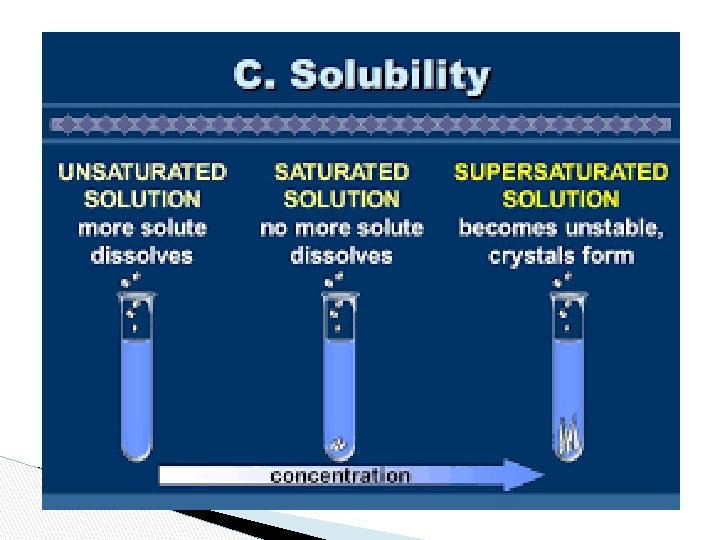
Saturated Solution: A solution which has dissolved the maximum amount of solute at a specific temperature. - It can’t dissolve anymore - Usually some solute visible on the bottom
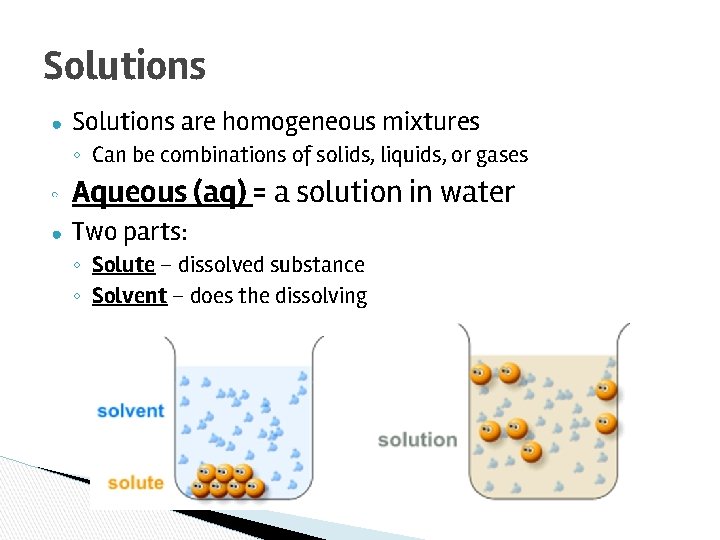
Solutions ● Solutions are homogeneous mixtures ◦ Can be combinations of solids, liquids, or gases ◦ Aqueous (aq) = a solution in water ● Two parts: ◦ Solute – dissolved substance ◦ Solvent – does the dissolving
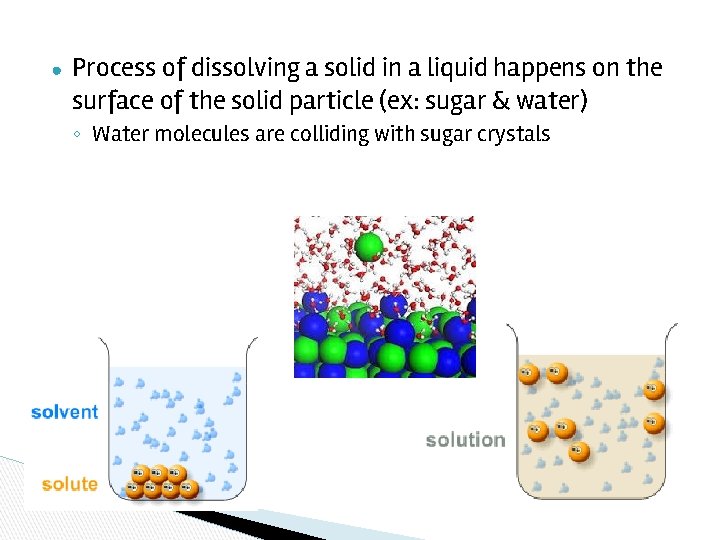
● Process of dissolving a solid in a liquid happens on the surface of the solid particle (ex: sugar & water) ◦ Water molecules are colliding with sugar crystals

How can I speed up the process of dissolving? ● Stirring ◦ Fresh solvent is continuously brought in contact with the surface of the solute. ◦ Stirring only affects rate of dissolving, not the amount dissolved. ● Increase Temperature ◦ Solvent particles move faster and collide with solute particles with more energy and more often. ● Decrease particle size ◦ If solute particles are smaller, they have a higher surface area to interact with the solvent.
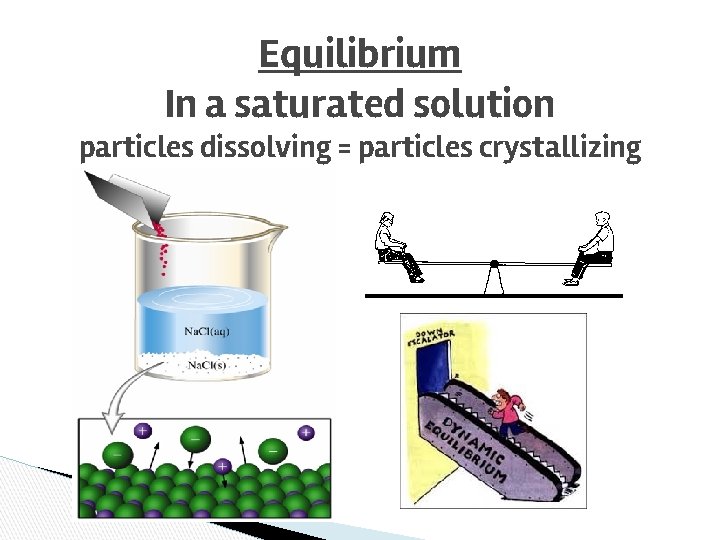
Equilibrium In a saturated solution particles dissolving = particles crystallizing
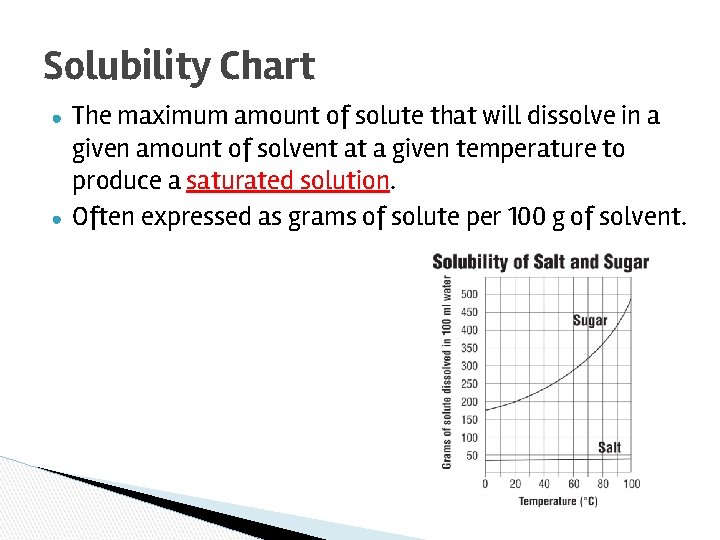
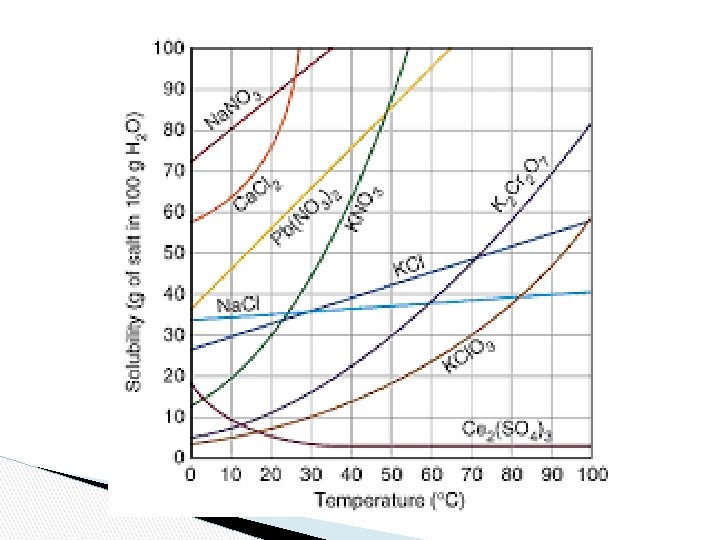
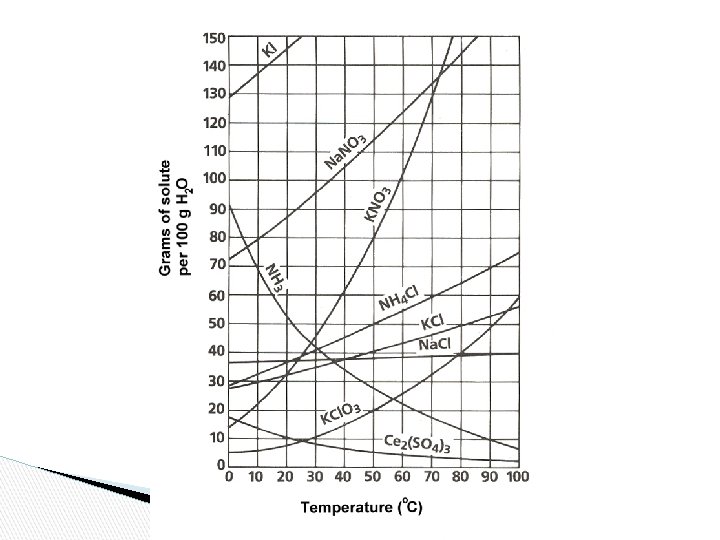
Solubility Chart ● ● The maximum amount of solute that will dissolve in a given amount of solvent at a given temperature to produce a saturated solution. Often expressed as grams of solute per 100 g of solvent.
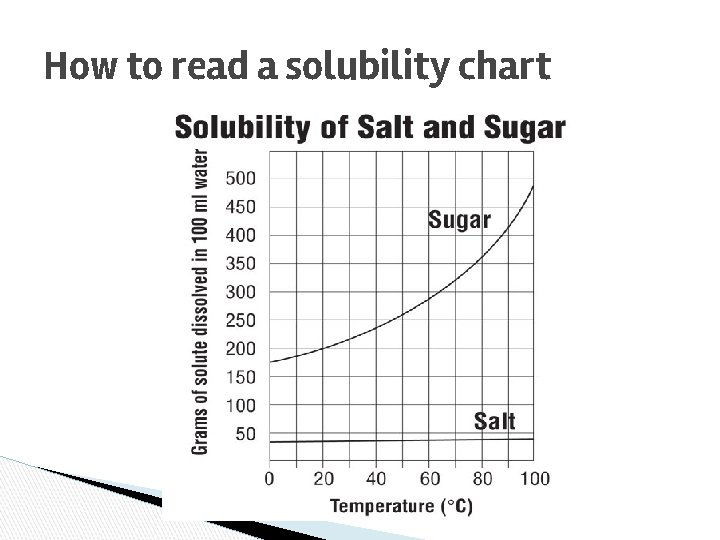
How to read a solubility chart
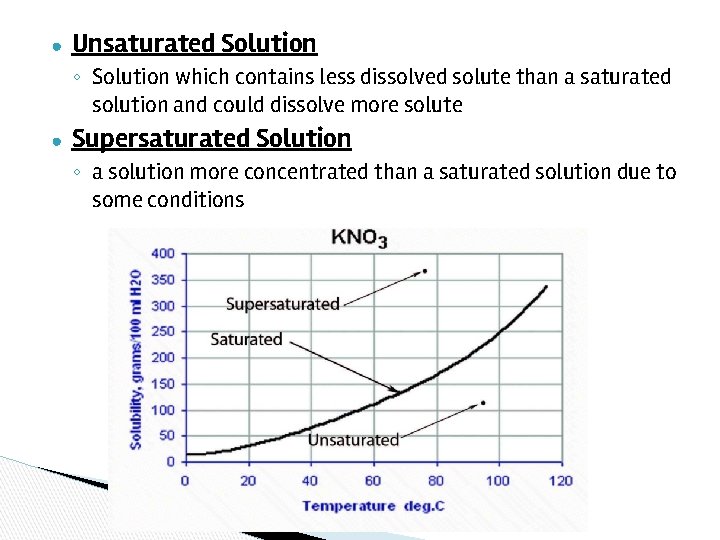
● Unsaturated Solution ◦ Solution which contains less dissolved solute than a saturated solution and could dissolve more solute ● Supersaturated Solution ◦ a solution more concentrated than a saturated solution due to some conditions
- Slides: 13