Solutions and Equilibrium Heterogeneous Equilibrium Reaction occurs in

Solutions and Equilibrium

Heterogeneous Equilibrium • Reaction occurs in more than one phase • KEQ = Products Reactants • Solids and liquids are not included in the equilibrium constant expression for heterogeneous equilibrium Fe. O(s) + CO(g) ⇌ Fe(s) + CO 2(g) KEQ = [CO 2] [CO]
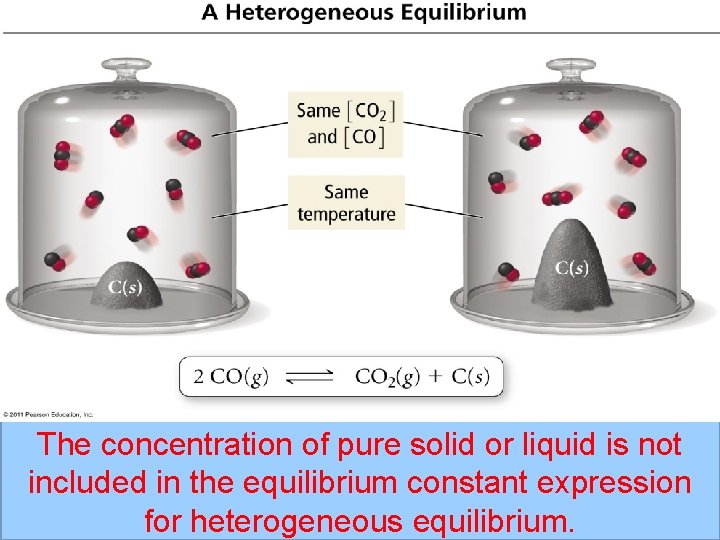
The concentration of pure solid or liquid is not included in the equilibrium constant expression for heterogeneous equilibrium.

Solubility Product Constant • The equilibrium constant for the solubility equilibrium of a slightly soluble ionic compound Ba. SO 4(s) Ba 2+(aq) + SO 42 -(aq) Ksp = [Ba 2+] [SO 42 - ] Pb. I 2 Pb 2+(aq) + 2 I-(aq) Ksp = [Pb 2+] [I-]2

Solubility Product Constant ** If Ksp is small = low solubility Ba. SO 4 Ksp = 1. 1 X 10 -10 Ca. SO 4 Ksp = 2. 4 X 10 -5 Fe(OH)2 Ksp = 7. 9 X 10 -16 Which is most soluble? Ca. SO 4 Which is least soluble? Fe(OH)2
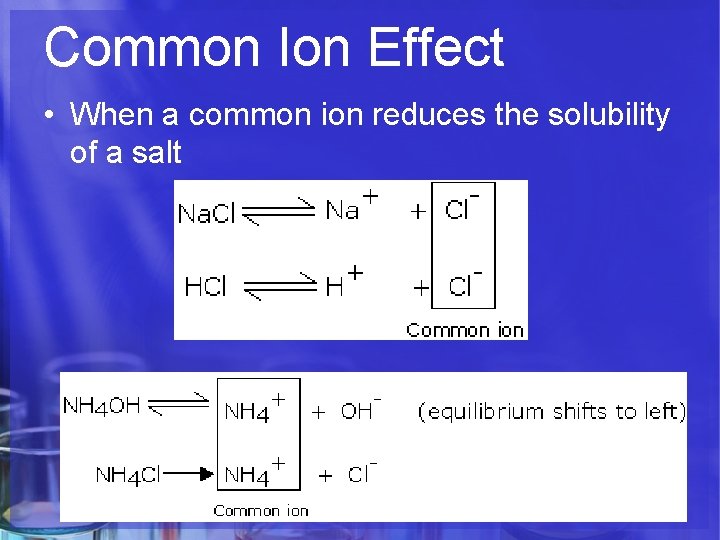
Common Ion Effect • When a common ion reduces the solubility of a salt

What is the common ion? Cl. Which direction is equilibrium driven? Left – forming more undissolved Na. Cl

Learning Check • If KBr is added to a solution containing Pb. Br 2(aq), will more Pb. Br 2 solidify? • Yes • If KBr is added to a solution containing Na. Cl, will more Na. Cl precipitate? • No, no common ion

Reactions that go to Completion • KEQ is large • A gas leaves the system 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) • Water is formed HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) • A precipitate is made (Table F) Ag. NO 3(aq) + Na. Cl(aq) Na. NO 3(aq) + Ag. Cl(s)
- Slides: 9