Solutions and Colligative Properties Solutions are homogeneous mixtures















- Slides: 41

Solutions and Colligative Properties Solutions are homogeneous mixtures of two or more substances in a single phase. Solute – substance being dissolved Solvent – dissolving medium

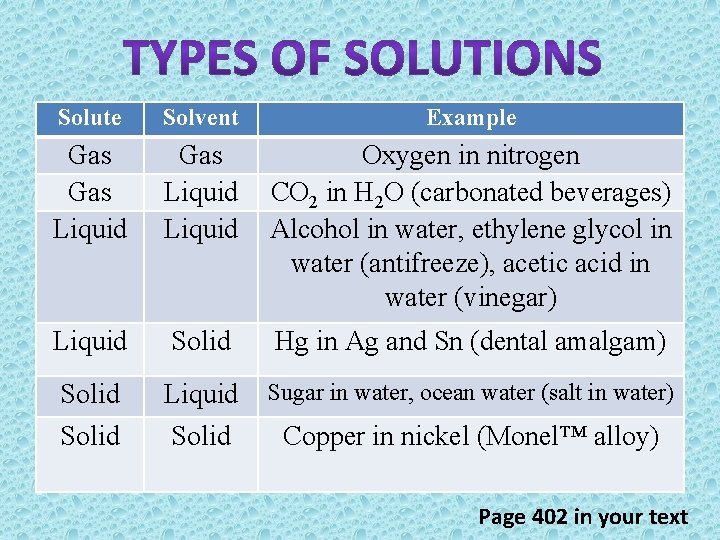
Solute Solvent Example Gas Liquid Oxygen in nitrogen CO 2 in H 2 O (carbonated beverages) Alcohol in water, ethylene glycol in water (antifreeze), acetic acid in water (vinegar) Liquid Solid Hg in Ag and Sn (dental amalgam) Solid Liquid Solid Sugar in water, ocean water (salt in water) Copper in nickel (Monel™ alloy) Page 402 in your text


Heterogeneous mixtures are suspensions and colloids. Brownian motion is the erratic movement of colloid particles. The Tyndall effect (named after John Tyndall) is when light is scattered by colloidal particles dispersed in a transparent medium. For example: car headlights in fog. http: //www. chm. bris. ac. uk/webprojects 2002/pdavies/T yndall. jpg

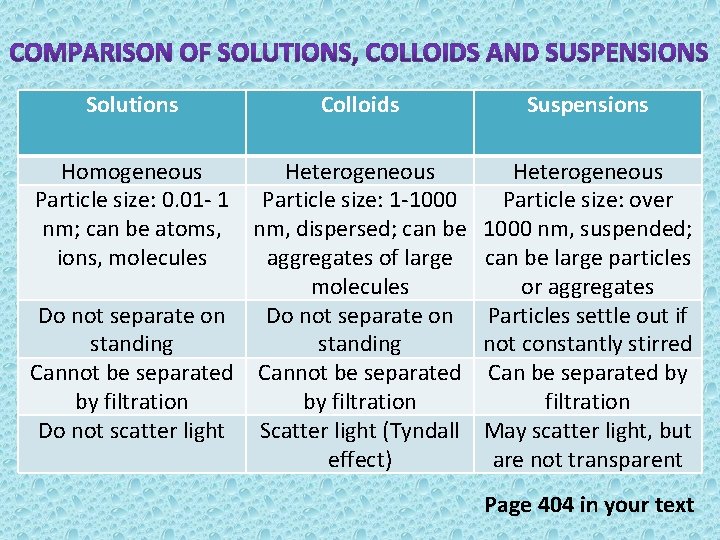
Solutions Colloids Suspensions Homogeneous Heterogeneous Particle size: 0. 01 - 1 Particle size: 1 -1000 Particle size: over nm; can be atoms, nm, dispersed; can be 1000 nm, suspended; ions, molecules aggregates of large can be large particles molecules or aggregates Do not separate on Particles settle out if standing not constantly stirred Cannot be separated Can be separated by by filtration Do not scatter light Scatter light (Tyndall May scatter light, but effect) are not transparent Page 404 in your text

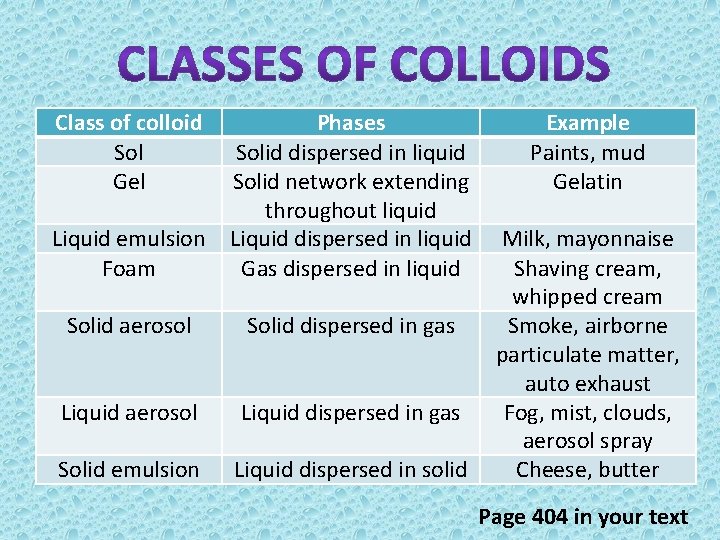
Class of colloid Sol Gel Liquid emulsion Foam Phases Solid dispersed in liquid Solid network extending throughout liquid Liquid dispersed in liquid Gas dispersed in liquid Solid aerosol Solid dispersed in gas Liquid aerosol Liquid dispersed in gas Solid emulsion Liquid dispersed in solid Example Paints, mud Gelatin Milk, mayonnaise Shaving cream, whipped cream Smoke, airborne particulate matter, auto exhaust Fog, mist, clouds, aerosol spray Cheese, butter Page 404 in your text

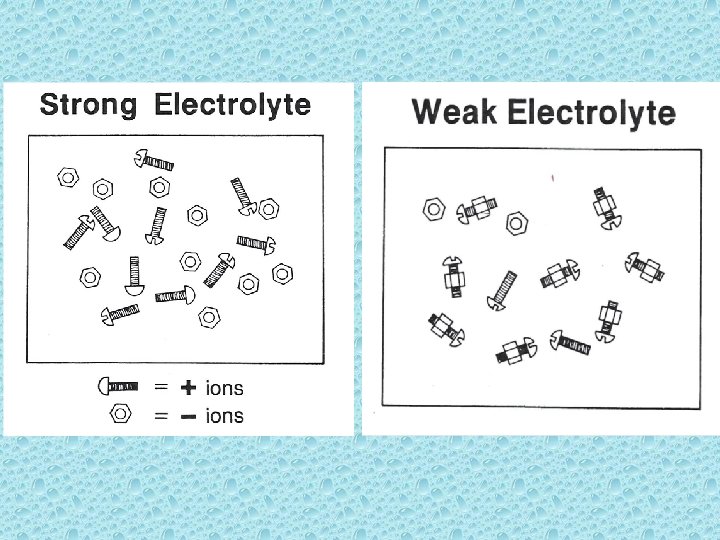
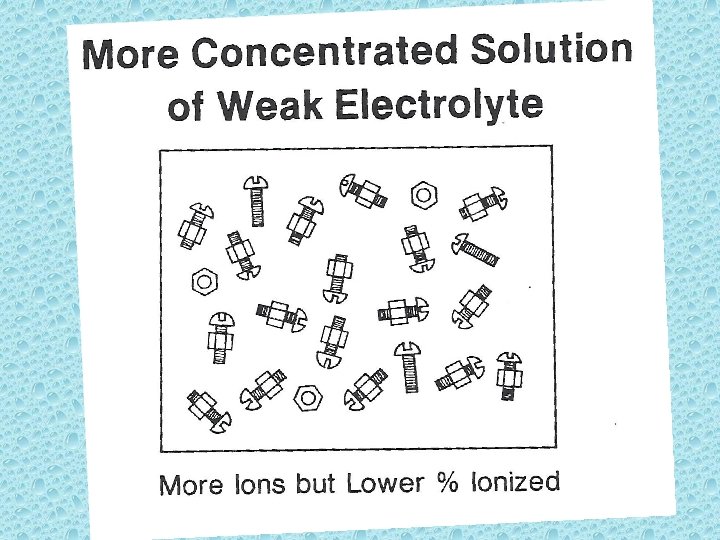
Solutes are classified according to the extent they dissociate into ions in aqueous solutions. • Strong electrolyte: substance that dissolves in water to give a solution that conducts electricity. (i. e. salt – Na. Cl) Weak electrolyte: less than 50% of dissolved solute exists as ions. (i. e. – acetic acid[vinegar])



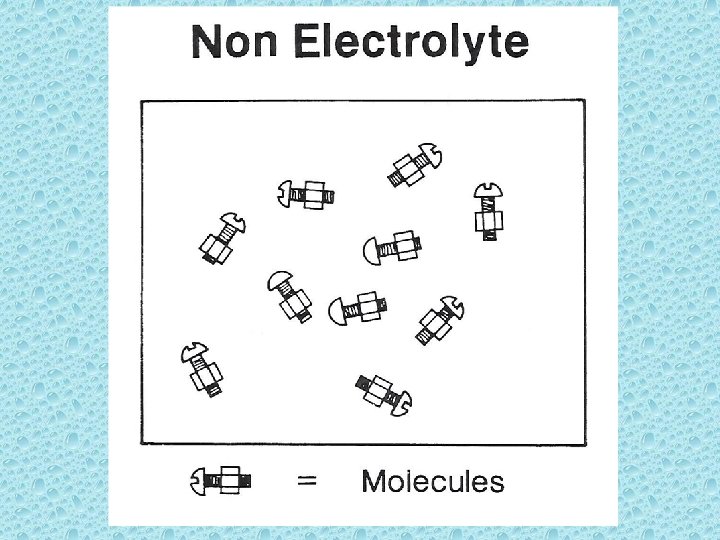
• Nonelectrolyte: < 0. 01% exists as ions; does not conduct electricity when solute is dissolved in water. (i. e. sugar – C 12 H 22 O 11)


Factors that affect the rate of dissolution: –stirring –temperature –surface area –Amount of solute already dissolved in solution

• Solubility is the maximum quantity of solute that can be dissolved in a given quantity of solvent at a particular temperature. It is usually expressed in g of solute per 100 g of solvent at a given temperature. • The three interactions that determine the solubility are solute-solute, solutesolvent, and solvent-solvent interactions.

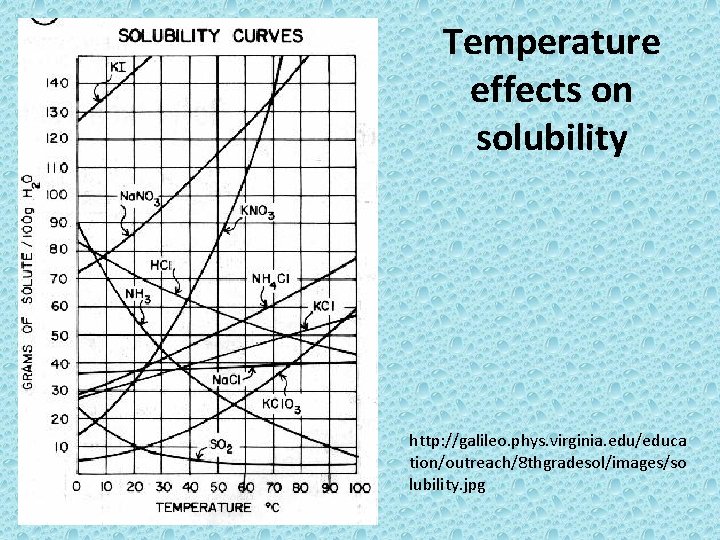
Temperature effects on solubility http: //galileo. phys. virginia. edu/educa tion/outreach/8 thgradesol/images/so lubility. jpg

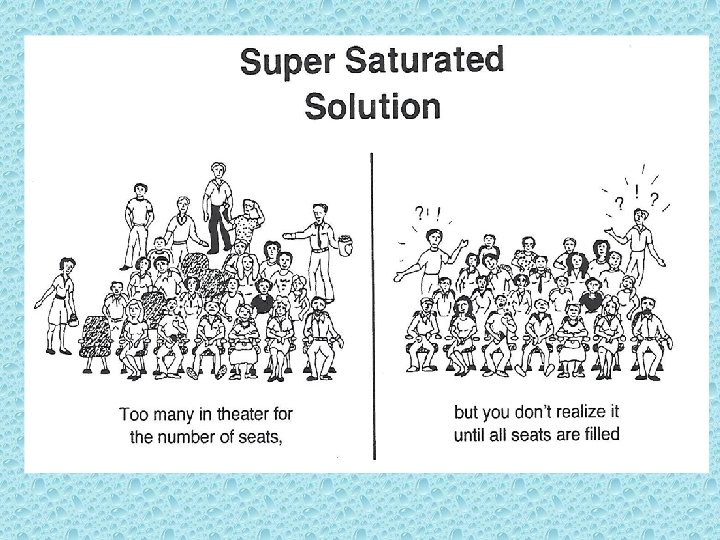
• Solution equilibrium is the physical state in which the opposing processes of dissolution and crystallization of a solute occur at equal rates. • Saturated solution: a solution that contains the maximum amount of dissolved solute. • Unsaturated solution: a solution that contains less than a saturated solution under the current conditions. • Supersaturated solution: a solution that contains more dissolved solute than a saturated solution contains under the same conditions.


• Nature of solute and solvent (polarity, etc. ) Solute-solvent interactions - “like dissolves like” • Temperature (for gases an increase in T decreases solubility). For the majority of solids in liquids, increasing T increases their solubility. The degree of solubility can differ greatly and in some cases, even decrease a solid’s solubility. • pressure (for solids and liquids has no effect; for gases an increase in P increases solubility) gas + solvent solution

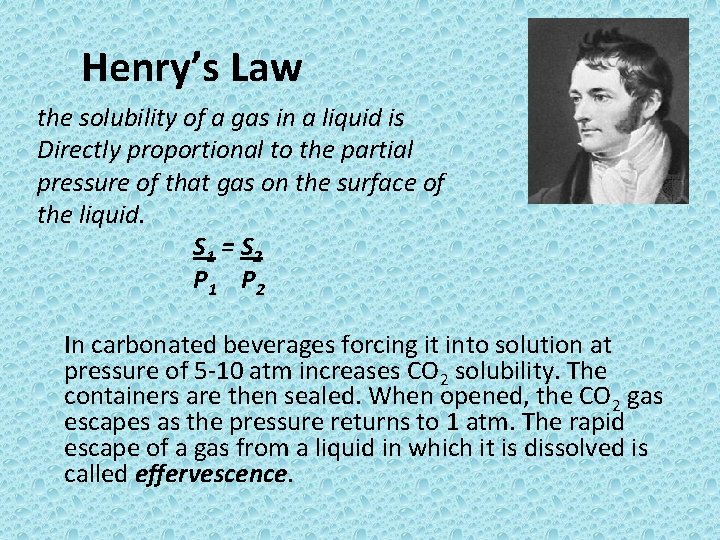
Henry’s Law the solubility of a gas in a liquid is Directly proportional to the partial pressure of that gas on the surface of the liquid. S 1 = S 2 P 1 P 2 In carbonated beverages forcing it into solution at pressure of 5 -10 atm increases CO 2 solubility. The containers are then sealed. When opened, the CO 2 gas escapes as the pressure returns to 1 atm. The rapid escape of a gas from a liquid in which it is dissolved is called effervescence.

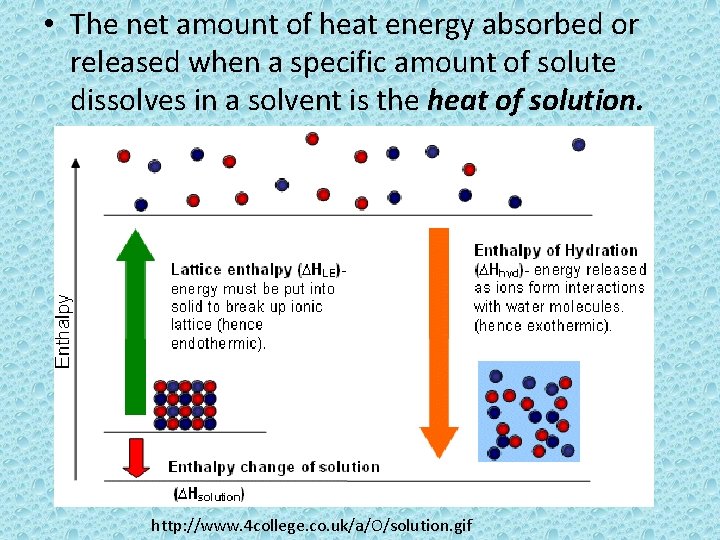
• The net amount of heat energy absorbed or released when a specific amount of solute dissolves in a solvent is the heat of solution. http: //www. 4 college. co. uk/a/O/solution. gif

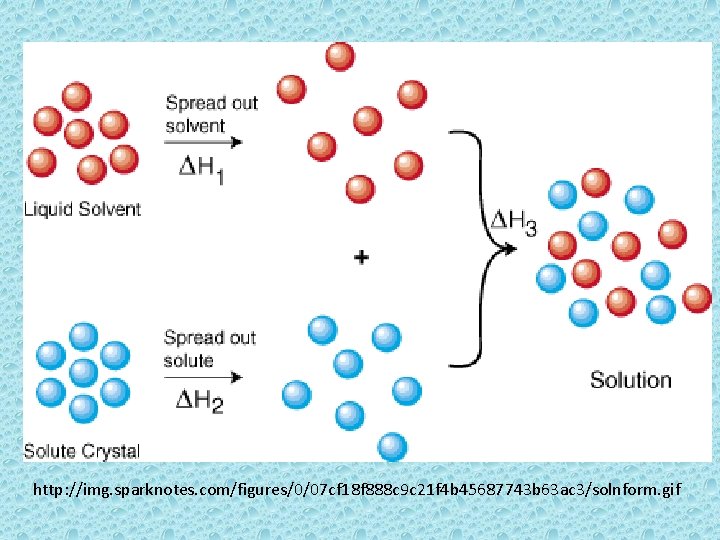
http: //img. sparknotes. com/figures/0/07 cf 18 f 888 c 9 c 21 f 4 b 45687743 b 63 ac 3/solnform. gif

When two liquids dissolve in each other at all proportions they are said to be miscible (i. e. alcohol and water); when they do not they are called immiscible (i. e. water and oil). You can separate immiscible liquids with a piece of equipment called a separatory funnel (shown at right http: //images. scran. ac. uk/RB/images/thumb/09343276. jpg

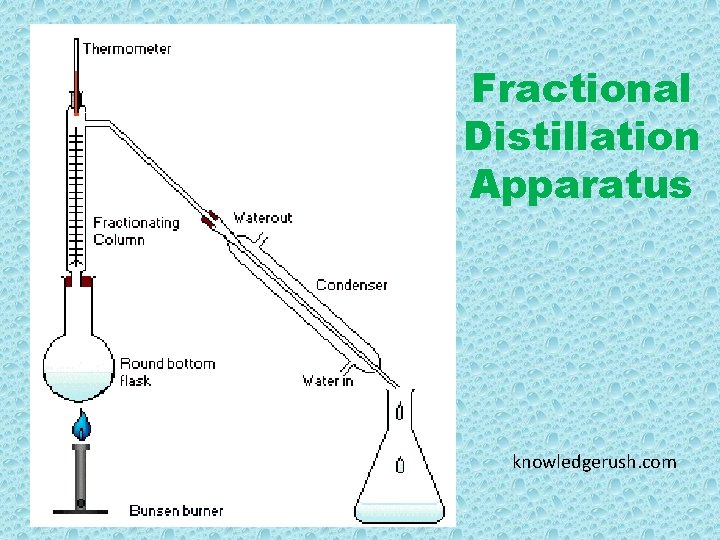
Fractional Distillation Apparatus knowledgerush. com

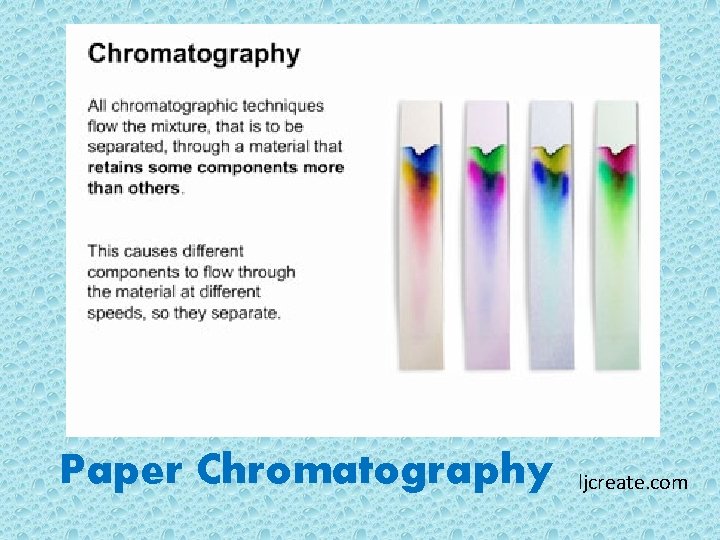
Paper Chromatography ljcreate. com

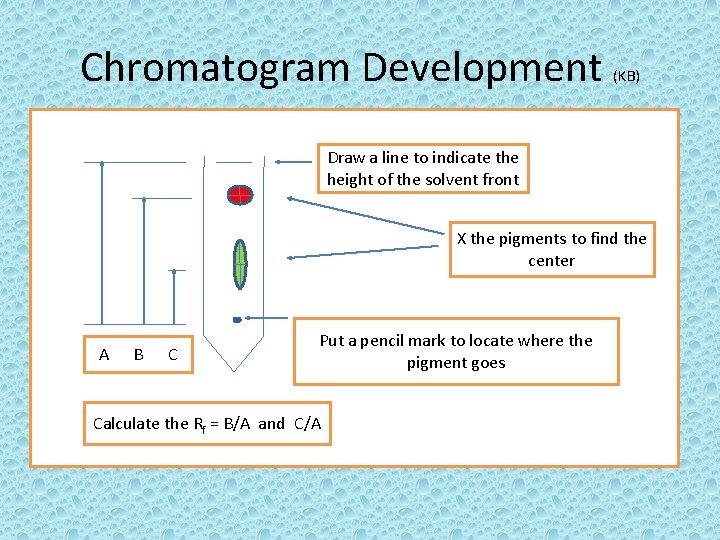
Chromatogram Development (KB) Draw a line to indicate the height of the solvent front X the pigments to find the center A B C Put a pencil mark to locate where the pigment goes Calculate the Rf = B/A and C/A

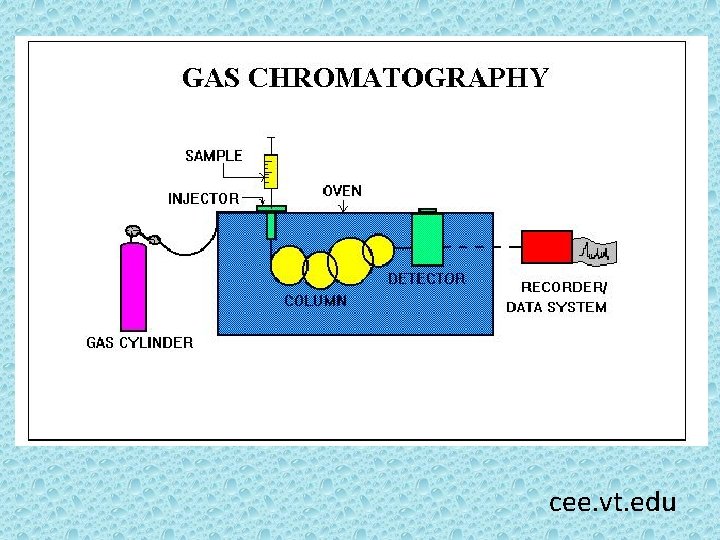
Gas chromatography (GC) is an analytical technique for separating compounds based primarily on their volatilities. Compounds move through a GC column as gases. The compounds partition between a stationary phase, which can be either solid or liquid, and a mobile phase (gas). The differential partitioning into the stationary phase allows the compounds to be separated in time and space. http: //www. cee. vt. edu/ewr/environmental/teach/smprimer/ gc/gc. html

cee. vt. edu

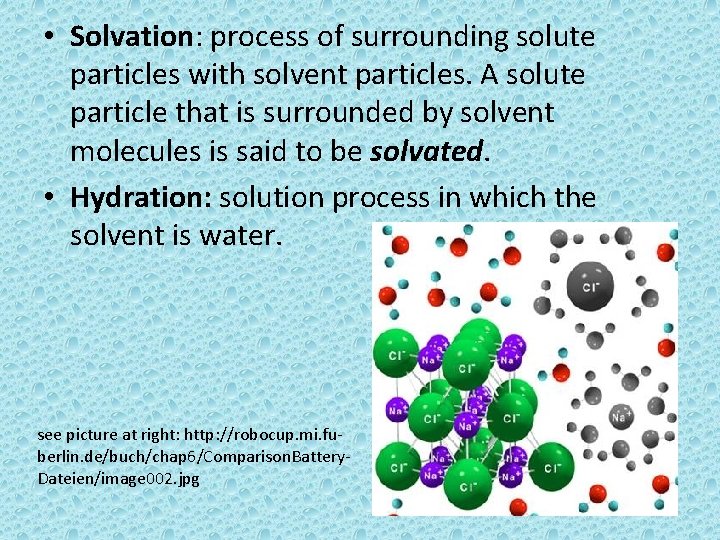
• Solvation: process of surrounding solute particles with solvent particles. A solute particle that is surrounded by solvent molecules is said to be solvated. • Hydration: solution process in which the solvent is water. see picture at right: http: //robocup. mi. fuberlin. de/buch/chap 6/Comparison. Battery. Dateien/image 002. jpg

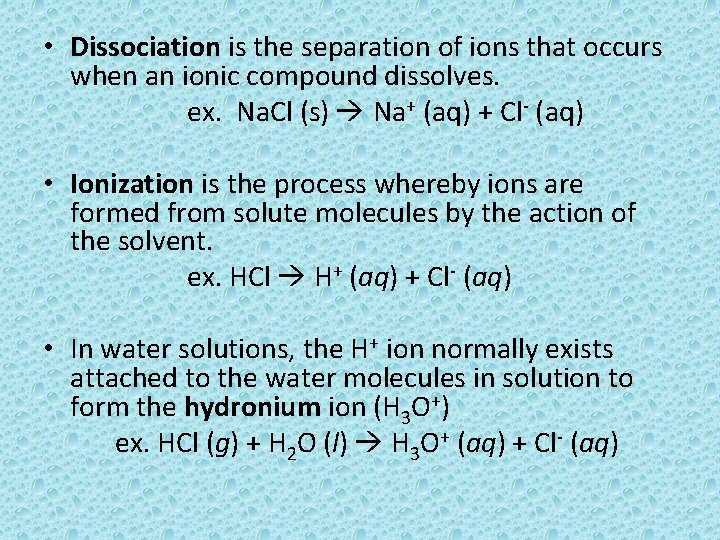
• Dissociation is the separation of ions that occurs when an ionic compound dissolves. ex. Na. Cl (s) Na+ (aq) + Cl- (aq) • Ionization is the process whereby ions are formed from solute molecules by the action of the solvent. ex. HCl H+ (aq) + Cl- (aq) • In water solutions, the H+ ion normally exists attached to the water molecules in solution to form the hydronium ion (H 3 O+) ex. HCl (g) + H 2 O (l) H 3 O+ (aq) + Cl- (aq)

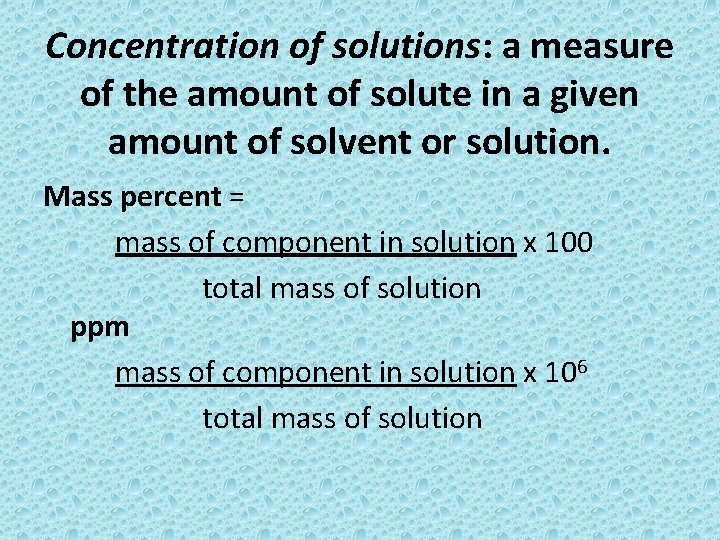
Concentration of solutions: a measure of the amount of solute in a given amount of solvent or solution. Mass percent = mass of component in solution x 100 total mass of solution ppm mass of component in solution x 106 total mass of solution

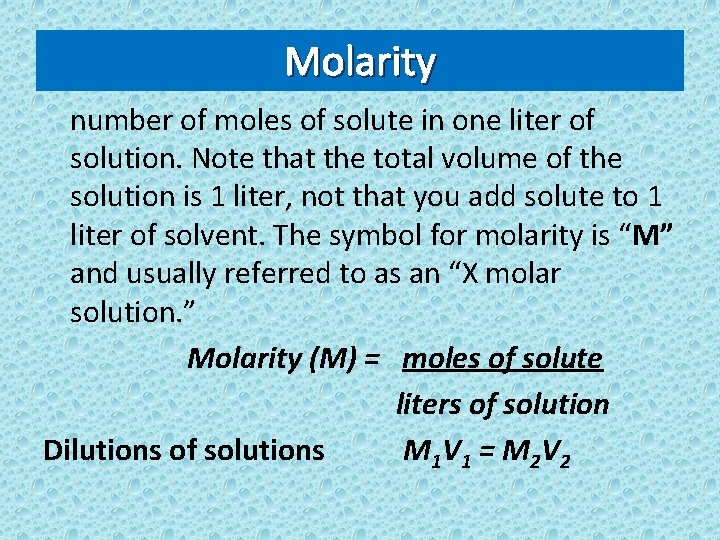
Molarity number of moles of solute in one liter of solution. Note that the total volume of the solution is 1 liter, not that you add solute to 1 liter of solvent. The symbol for molarity is “M” and usually referred to as an “X molar solution. ” Molarity (M) = moles of solute liters of solution Dilutions of solutions M 1 V 1 = M 2 V 2

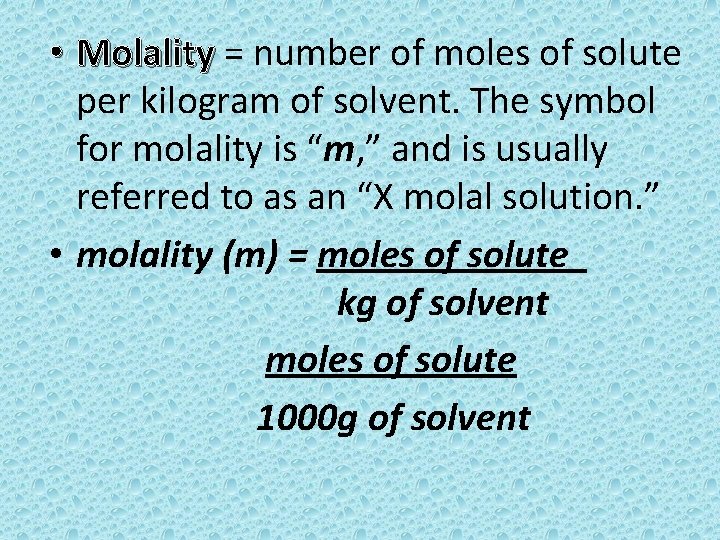
• Molality = number of moles of solute Molality per kilogram of solvent. The symbol for molality is “m, ” and is usually referred to as an “X molal solution. ” • molality (m) = moles of solute kg of solvent moles of solute 1000 g of solvent

Typically when two aqueous solutions are combined and one of the products is minimally soluble a PPT is formed

http: //boomeria. org/chemlectures/textass 2/table. A-7. jpg Table 1 in your textbook page 437; Table A-12 (appendix) page 860

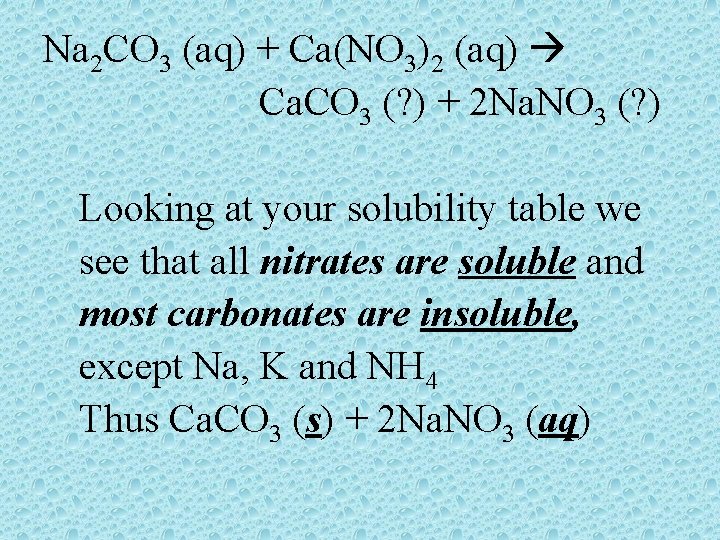
Na 2 CO 3 (aq) + Ca(NO 3)2 (aq) Ca. CO 3 (? ) + 2 Na. NO 3 (? ) Looking at your solubility table we see that all nitrates are soluble and most carbonates are insoluble, except Na, K and NH 4 Thus Ca. CO 3 (s) + 2 Na. NO 3 (aq)

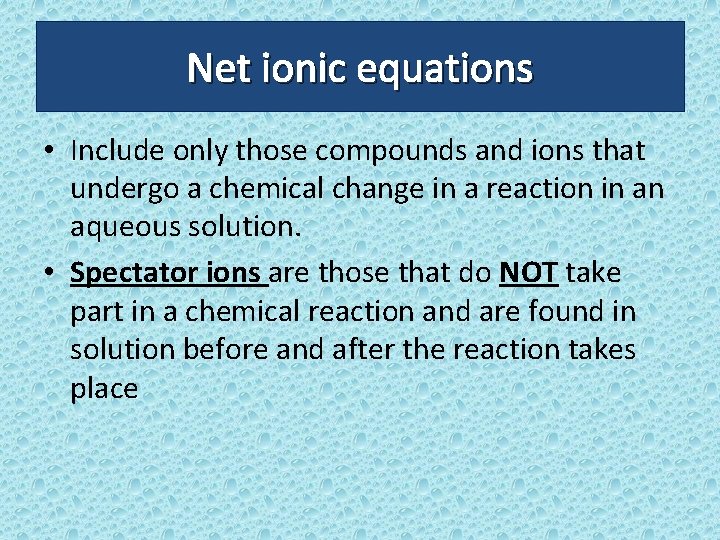
Net ionic equations • Include only those compounds and ions that undergo a chemical change in a reaction in an aqueous solution. • Spectator ions are those that do NOT take part in a chemical reaction and are found in solution before and after the reaction takes place

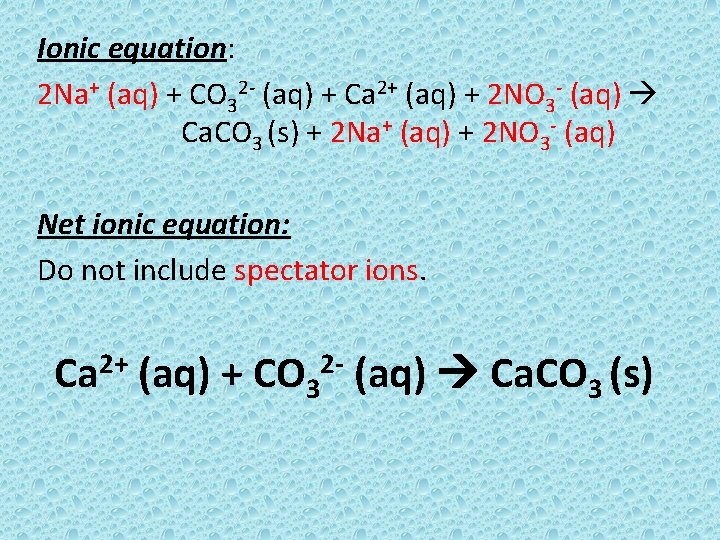
Ionic equation: 2 Na+ (aq) + CO 32 - (aq) + Ca 2+ (aq) + 2 NO 3 - (aq) Ca. CO 3 (s) + 2 Na+ (aq) + 2 NO 3 - (aq) Net ionic equation: Do not include spectator ions. Ca 2+ (aq) + CO 32 - (aq) Ca. CO 3 (s)

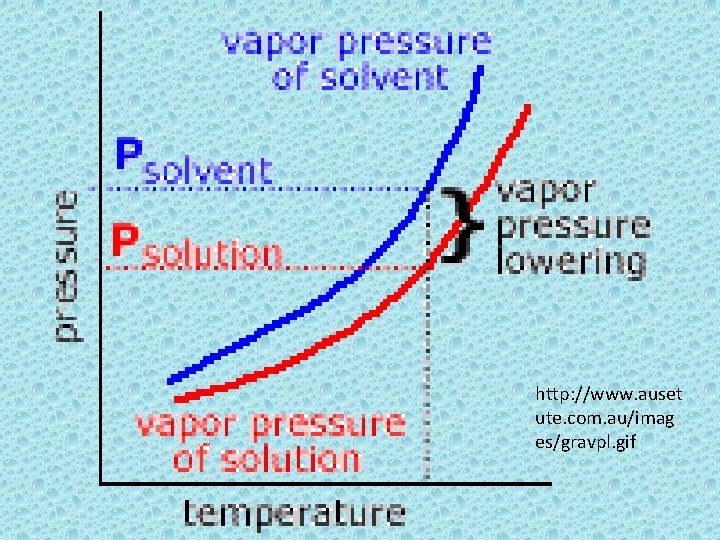
Colligative Properties • properties that depend on the concentration of solute particles but not on their identity. • Vapor pressure lowering. Boiling point is higher and freezing point of a solution is lower than that of a pure solvent. This is due to the presence of nonvolatile solutes. These are substances that have little tendency to become a gas under existing conditions.

http: //www. auset ute. com. au/imag es/gravpl. gif

• Freezing point depression, ∆Tf, is the difference between the freezing points of the pure solvent and a solution of a non-electrolyte in that solvent, and it is directly proportional to the molal concentration of the solution. ∆Tf = Kf m • Kf = the molal freezing-point constant is the freezing-point depression of the solvent in a 1 molal solution of a nonvolatile, nonelectrolyte solute. The value for this constant depends on the solvent used. (See a reference table for specific values)

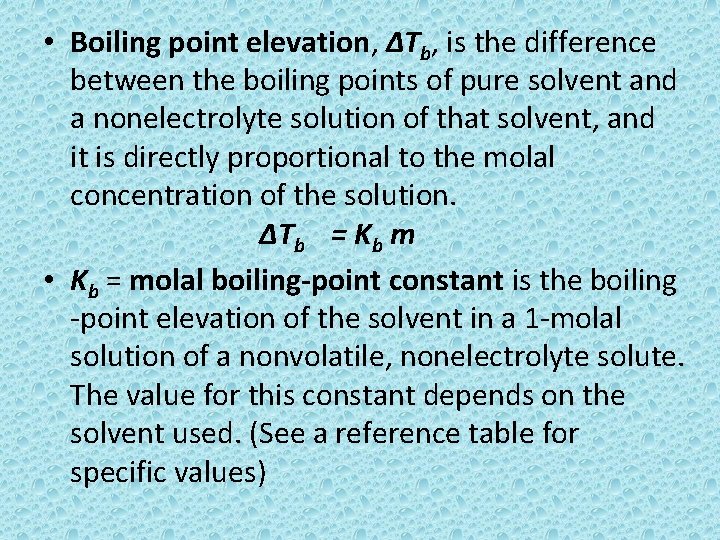
• Boiling point elevation, ∆Tb, is the difference between the boiling points of pure solvent and a nonelectrolyte solution of that solvent, and it is directly proportional to the molal concentration of the solution. ∆Tb = Kb m • Kb = molal boiling-point constant is the boiling -point elevation of the solvent in a 1 -molal solution of a nonvolatile, nonelectrolyte solute. The value for this constant depends on the solvent used. (See a reference table for specific values)

Electrolytes and colligative properties • Keeping in mind that colligative properties depend on the number of solute particles, not their identity, we must know the extent to which electrolytes dissociate. • The higher the degree of dissociation, the more moles of ions we have in solution, thus increasing the net effect.