Solutions Acids Bases Unit 9 What is a

Solutions, Acids & Bases Unit 9

What is a solution? Solution – a mixture where the components are uniformly intermingled. Homogeneous Solutions are made up of a solvent and solute. • Solute – substance that is dissolved • Solvent – substance in which materials are dissolved in. The medium in which the solute is dissolved in.

Solution Composition The solubility of a solute is limited. • Saturated solution – contains as much solute as will dissolve at that temperature • Unsaturated solution – has not reached the limit of solute that will dissolve • Oversaturated solution – contains too much solute in which the rest of solute remains on the bottom

Supersaturated solution – occurs when a solution is saturated at an elevated temperature and then allowed to cool but all of the solid remains dissolved • Contains more dissolved solid than a saturated solution at that temperature • Unstable – adding a crystal causes precipitation

• Amounts of substances can vary in different solutions. – Specify the amounts of solvent and solutes – Qualitative measures of concentration • concentrated – relatively large amount of solute • dilute – relatively small amount of solute
B. Solution Composition: An Introduction Which solution is more concentrated?
B. Solution Composition: An Introduction Which solution is more concentrated?

C. Factors Affecting the Rate of Dissolving Surface area Stirring Temperature
Solubility Curves Graphs that illustrates how much of solute will dissolve at any given temperature.
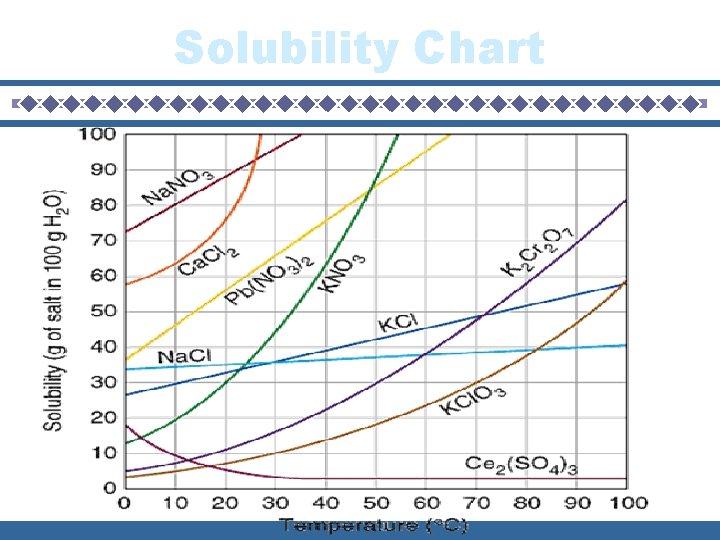
Solubility Chart

Questions How many grams of KCl. O 3 is diluted in water at 90 degrees? How many grams of K 2 Cr 2 O 7 is diluted in water at 60 degrees? How many grams of Pb(NO 3)2 is diluted in water at 10 degrees? 70 g of Ca. Cl 2 will dissolve at what temperature to form a saturated solution? 40 g of Na. Cl will dissolve at what temperature to form a saturated solution? 90 g of KNO 3 will dissolve at what temperature to form a saturated solution? At 30 degrees, you dissolve 25 grams of KNO 3 in water. How much more KNO 3 can you add to make it a saturated solution? At 10 degrees, you dissolve 25 grams of Na. NO 3 in water. How much more Na. NO 3 can you add to make it a saturated solution? At 90 degrees, you dissolve 10 grams of KCl. O 3 in water. How much more KCl. O 3 can you add to make it a saturated solution? Which salt has solubility values that are least affected by temperature? At 75 degrees, I attempted to dissolve 50 g of KCl. Is this a saturated, unsaturated, or supersaturated solution? At 40 degrees, I attempted to dissolve 50 g of Na. Cl. Is this a saturated, unsaturated, or supersaturated solution? At 55 degrees, I attempted to dissolve 50 g of Pb(NO 3)2. Is this a saturated, unsaturated, or supersaturated solution?
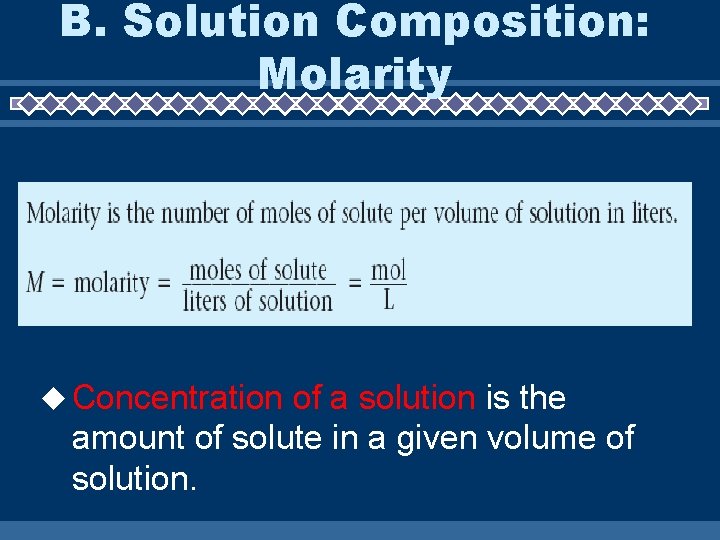
B. Solution Composition: Molarity Concentration of a solution is the amount of solute in a given volume of solution.
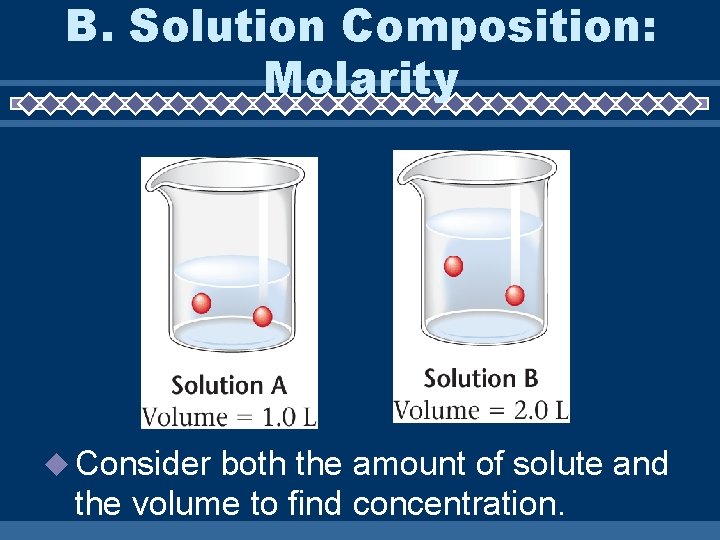
B. Solution Composition: Molarity Consider both the amount of solute and the volume to find concentration.
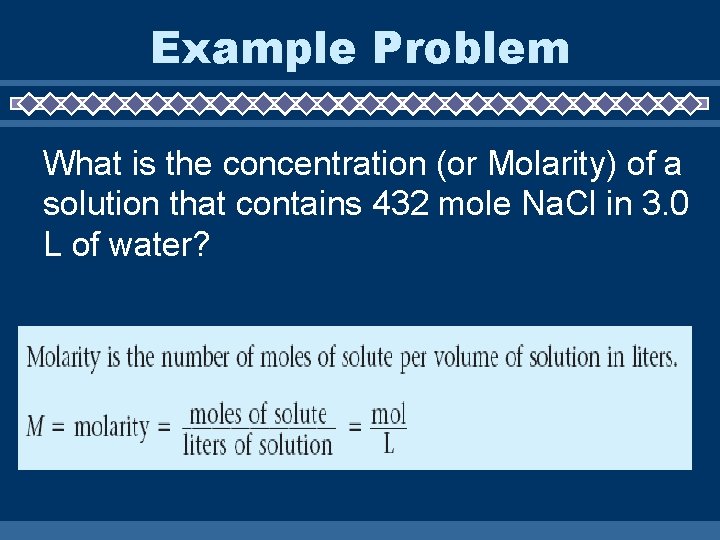
Example Problem What is the concentration (or Molarity) of a solution that contains 432 mole Na. Cl in 3. 0 L of water?


Example Problem What is the concentration of a solution that contains 11. 2 g of Li. Cl in a 0. 50 L solution? • Make sure that you convert the grams to moles before you find the molarity.

First, convert the grams to moles. 11. 2 g Li. Cl 1 mol Li. Cl 42. 39 g Li. Cl = 0. 264 mol Li. Cl Use the moles to find the molarity. = 0. 53 M
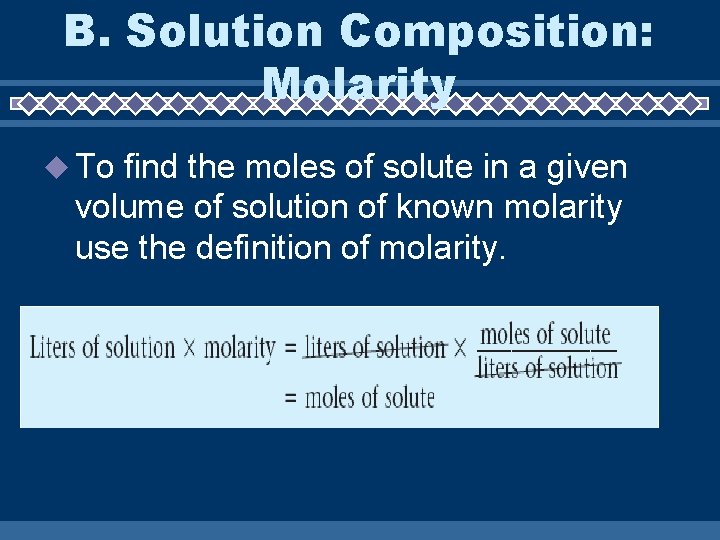
B. Solution Composition: Molarity To find the moles of solute in a given volume of solution of known molarity use the definition of molarity.

How many grams of HCl was mixed into a 3 L of a 9 M solution? Remember that:

How many grams of HCl was mixed into a 3 L of a 9 M solution? What do we know? • Concentration = 9 M • L of solution = 3 • Mols of solute = ?

How many grams of HCl was mixed into a 3 L of a 9 M solution? Solve for the moles of solute
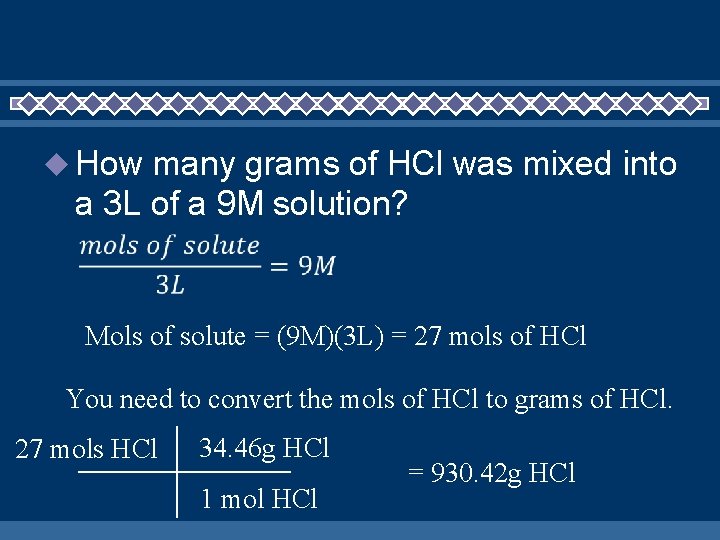
How many grams of HCl was mixed into a 3 L of a 9 M solution? Mols of solute = (9 M)(3 L) = 27 mols of HCl You need to convert the mols of HCl to grams of HCl. 27 mols HCl 34. 46 g HCl 1 mol HCl = 930. 42 g HCl

Practice Problems Calculate the molarity of a solution prepared by dissolving 11. 5 g of solid Na. OH in enough water to make 1. 50 L of solution. 2. 2. Calculate the molarity of a solution prepared by dissolving 1. 56 g of gaseous HCl into enough water to make 0. 0268 L of solution. 1.

Practice Problems 3. What is the volume of a 3. 2 M solution of H 2 SO 4 in a 0. 30 L beaker? 4. What is the mass (g) of a 1. 2 M solution of Ca. CO 3 in a 0. 50 L flask?

Dilution Water can be added to an aqueous solution to dilute the solution to a lower concentration. Only water is added in the dilution – the amount of solute is the same in both the original and final solution.

D. Dilution Diluting a solution • Transfer a measured amount of original solution to a flask containing some water. • Add water to the flask to the mark (with swirling) and mix by inverting the flask.

D. Dilution We can use a simple equation can be used in order to correctly dilute a solution to any concentration M 1 V 1 = M 2 V 2 M 1 = the molarity of the initial solution V 1 = volume of the initial solution M 2 = the molarity of the diluted solution V 2 = volume of the diluted solution
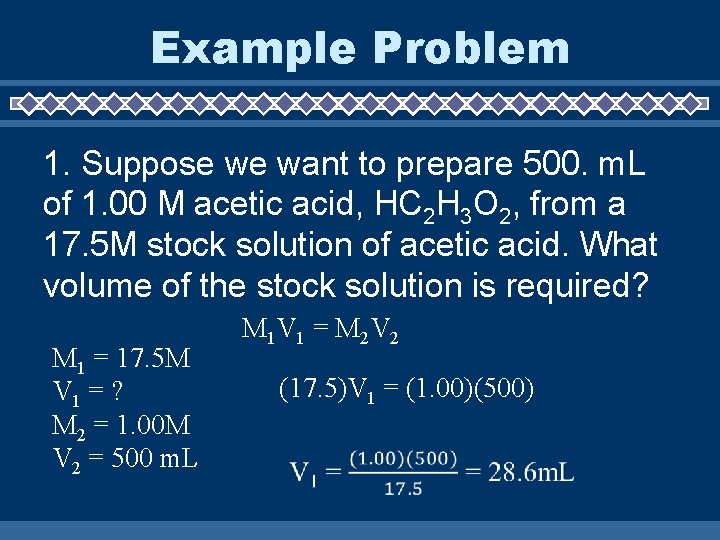
Example Problem 1. Suppose we want to prepare 500. m. L of 1. 00 M acetic acid, HC 2 H 3 O 2, from a 17. 5 M stock solution of acetic acid. What volume of the stock solution is required? M 1 = 17. 5 M V 1 = ? M 2 = 1. 00 M V 2 = 500 m. L M 1 V 1 = M 2 V 2 (17. 5)V 1 = (1. 00)(500)

Practice Problems What volume of 16 M sulfuric acid must be used to prepare 1. 5 L of a 0. 10 M H 2 SO 4 solution? 2. What is the concentration when you dilute 0. 3 L of 10 M of HCl in to a. 500 L solution? 6 M 3. What is the concentration of a 250 m. L solution that was used to make a 6 M 600 m. L solution of 2. 5 M of Na. Cl? 1.

Acids & Bases I. Introduction to Acids & Bases
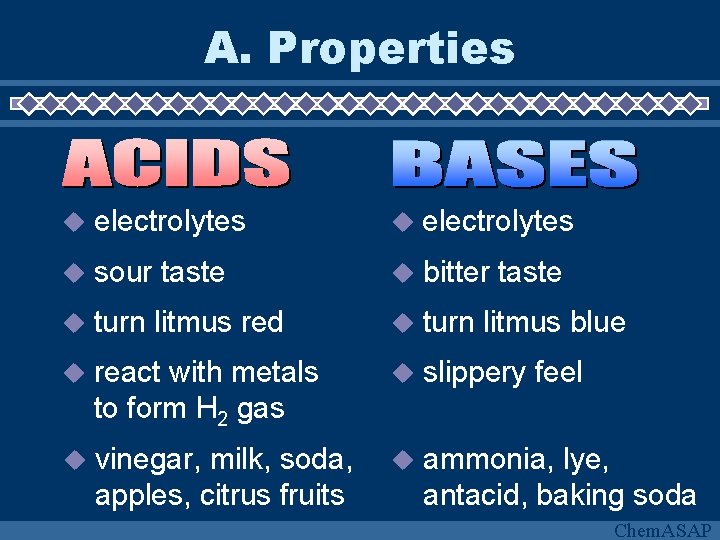
A. Properties electrolytes sour taste bitter taste turn litmus red turn litmus blue react with metals to form H 2 gas slippery feel vinegar, milk, soda, apples, citrus fruits ammonia, lye, antacid, baking soda Chem. ASAP

Acids (Svante Arrhenius’ Definition) Acids form hydrogen ions or hydronium ions when dissolved in water H+ H O+ 3
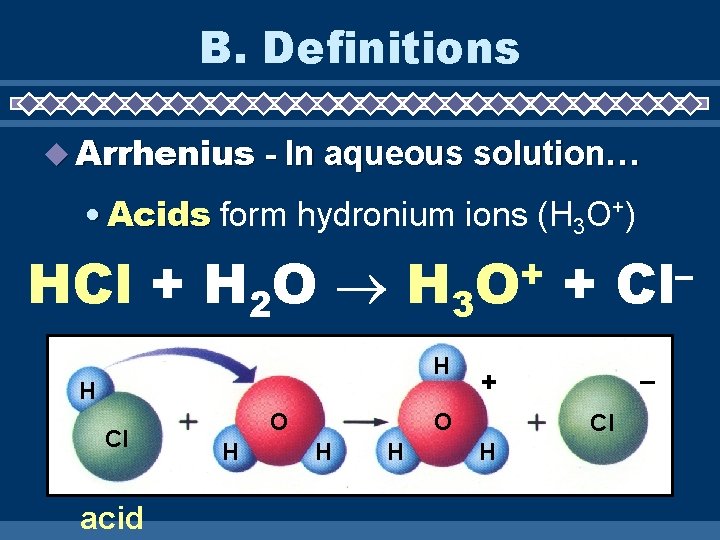
B. Definitions Arrhenius - In aqueous solution… • Acids form hydronium ions (H 3 O+) HCl + H 2 O H 3 H H Cl acid O H + O H – + O H + Cl H – Cl

Common Acids HCl Hydrochloric H SO 2 Sulfuric 4 HNO Nitric 3 H PO Phosphoric 3 4 H CO Carbonic 2 3
Acid Nomenclature Binary Compound – compound consisting of 2 elements. An acid binary compound consists of 2 elements in which one of them is ALWAYS hydrogen. • HF • HCl • HBr

Acid Nomenclature To name acid binary compounds • Add hydro- as a prefix • Use the root of the element and add – ic acid as a suffix

Acid Nomenclature HF • Hydrofluoric acid HCl • Hydrochloric acid H 2 S • Hydrosulfuric acid H 3 P • Hydrophosphuric acid

Acid Nomenclature Writing the name from chemical formulas • Figure out the charge of the anion • Hydrobromic acid – H+ and Br • Br has a negative 1 charge • Add enough H+ until the compound is neutral

Acid Nomenclature Hydronitric acid • H 3 N Hydrofluoric acid • HF Hydroselenic acid • H 2 Se
Acid Nomenclature Acid polyatomic compounds Remember polyatomic compounds are compounds that have a charge. When naming polyatomic acids… • DO NOT USE HYDRO- as preffix • Use either –ic acid or –ous acid
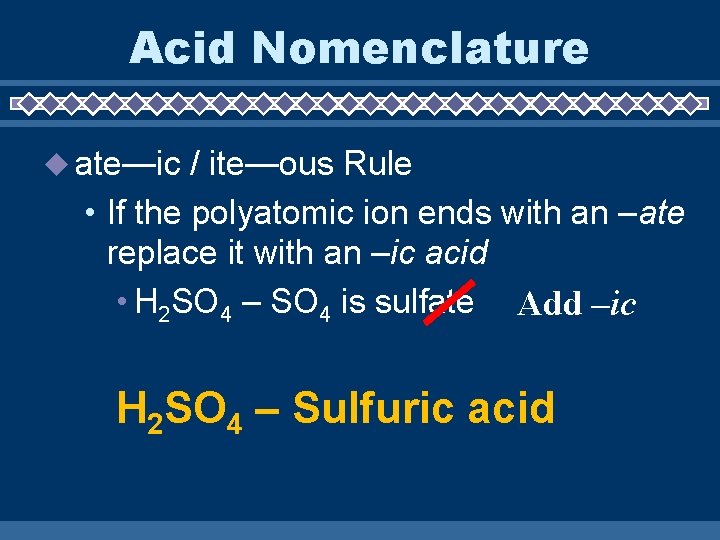
Acid Nomenclature ate—ic / ite—ous Rule • If the polyatomic ion ends with an –ate replace it with an –ic acid • H 2 SO 4 – SO 4 is sulfate Add –ic H 2 SO 4 – Sulfuric acid

Acid Nomenclature ate—ic / ite—ous Rule • If the polyatomic ion ends with an –ite then replace it with an –ous acid • HNO 2 – NO 2 is nitrite Add -ous HNO 2 – Nitrous acid

Example Problems 1. 2. 3. 4. 5. 6. 7. H 2 SO 3 H 3 PO 4 HCl. O 2 HC 2 H 3 O 2 H 2 Cr 2 O 7 H 2 CO 3 HNO 2 Sulfurous acid Phosphoric acid Chlorous acid Acetic acid Dichromic acid Carbonic acid Nitrous acid
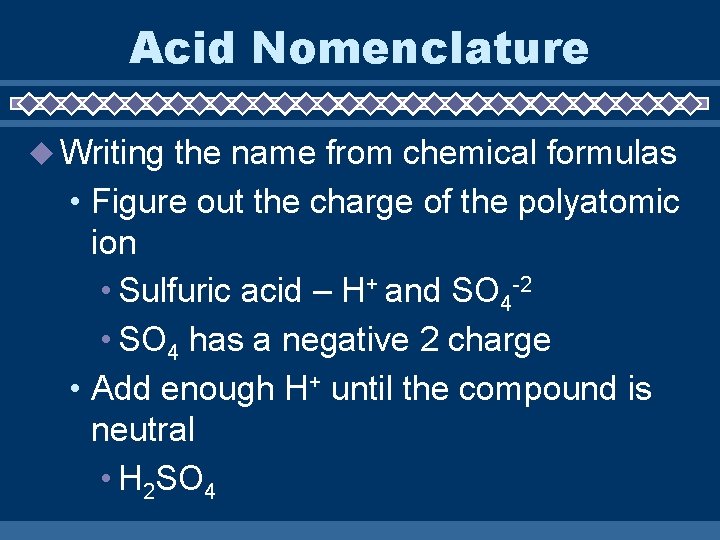
Acid Nomenclature Writing the name from chemical formulas • Figure out the charge of the polyatomic ion • Sulfuric acid – H+ and SO 4 -2 • SO 4 has a negative 2 charge • Add enough H+ until the compound is neutral • H 2 SO 4

Example Problems 1. 2. 3. 4. 5. Hypochlorous acid Perchloric acid Nitric acid Permanganic acid Sulfurous acid HCl. O 4 HNO 3 HMn. O 4 H 2 SO 3

More Example Problems 1. 2. 3. 4. 5. 6. 7. 8. HF Hydrochloric acid H 2 Se Chromic acid HCl. O 3 Chlorous acid HNO 3 H 3 N Hydrofluoric acid HCl Hydroselenic acid H 2 Cr. O 4 Chloric acid HCl. O 2 Nitric acid Hydronitric acid

Bases (Arrhenius’ Definition) Bases form hydroxide ions when dissolved in water OH
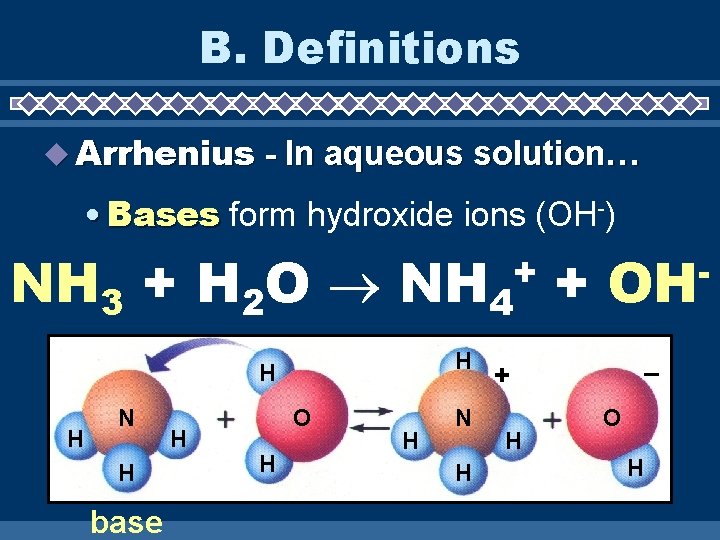
B. Definitions Arrhenius - In aqueous solution… • Bases form hydroxide ions (OH-) NH 3 + H 2 O NH 4 + + H H H N H base H O H H N H OH – + H O H

Common Bases Na. OH NH 4 Sodium Hydroxide OH Ammonium Hydroxide Ca(OH) 2 Calcium Hydroxide
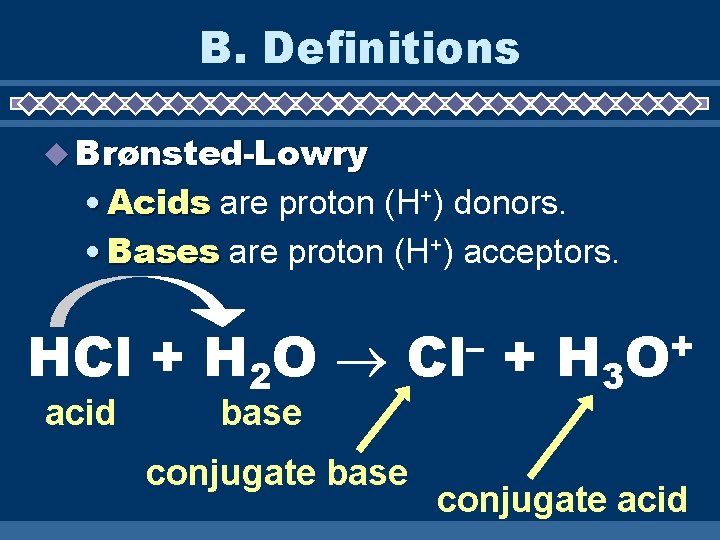
B. Definitions Brønsted-Lowry • Acids are proton (H+) donors. • Bases are proton (H+) acceptors. HCl + H 2 O acid – Cl base conjugate base + H 3 + O conjugate acid
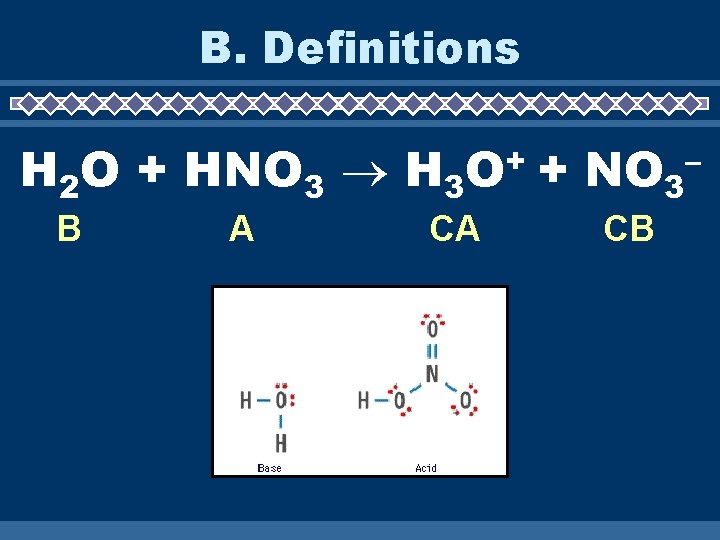
B. Definitions H 2 O + HNO 3 H 3 B A + O CA + NO 3 CB –
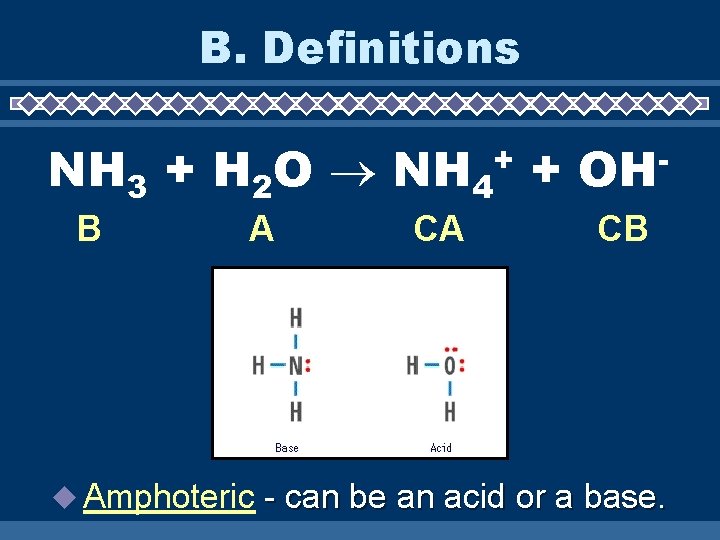
B. Definitions NH 3 + H 2 O NH 4 + + B A Amphoteric CA OH CB - can be an acid or a base.
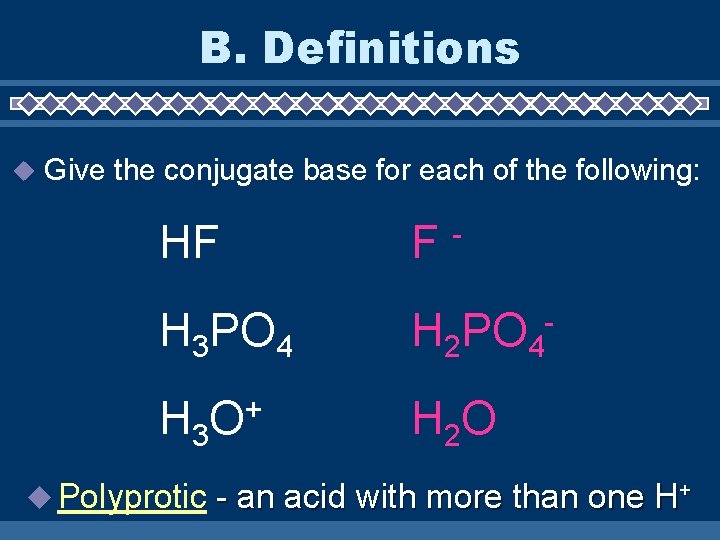
B. Definitions Give the conjugate base for each of the following: HF F H 3 PO 4 H 2 PO 4 - H 3 O + H 2 O Polyprotic - - an acid with more than one H+
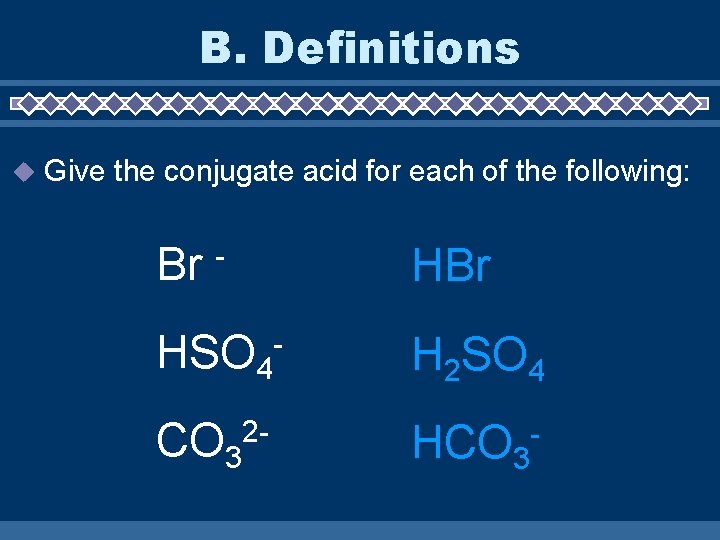
B. Definitions Give the conjugate acid for each of the following: Br - HBr HSO 4 - H 2 SO 4 CO 3 HCO 3 - 2 -
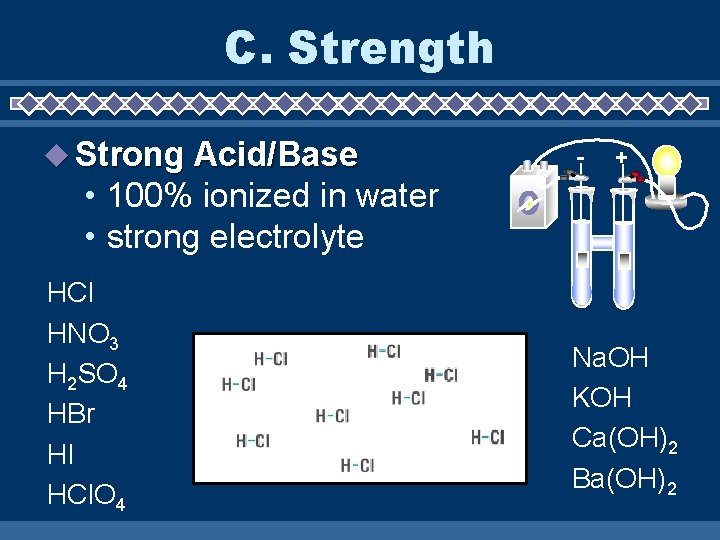
C. Strength Strong Acid/Base - + • 100% ionized in water • strong electrolyte HCl HNO 3 H 2 SO 4 HBr HI HCl. O 4 Na. OH KOH Ca(OH)2 Ba(OH)2
C. Strength Weak Acid/Base - + • does not ionize completely • weak electrolyte HF CH 3 COOH H 3 PO 4 H 2 CO 3 HCN NH 3
p. H
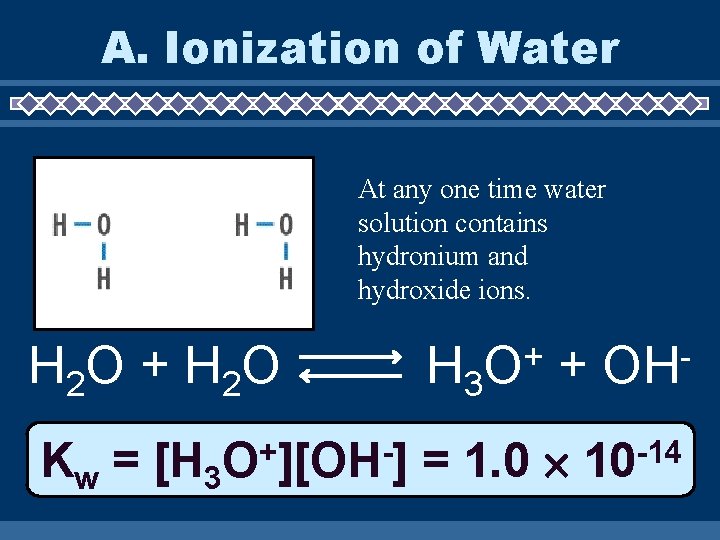
A. Ionization of Water At any one time water solution contains hydronium and hydroxide ions. H 2 O + H 2 O Kw = [H 3 + O ][OH ] H 3 + O + = 1. 0 OH -14 10

A. Ionization of Water Find the hydroxide ion concentration of 3. 0 10 -2 M HCl. [H 3 O+][OH-] = 1. 0 10 -14 [3. 0 10 -2][OH-] = 1. 0 10 -14 [OH-] = 3. 3 10 -13 M Acidic or basic? Acidic
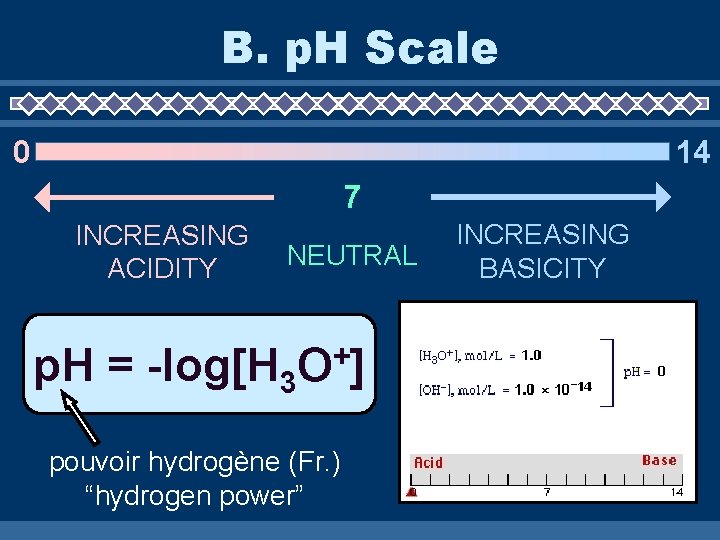
B. p. H Scale 14 0 7 INCREASING ACIDITY NEUTRAL p. H = -log[H 3 + O] pouvoir hydrogène (Fr. ) “hydrogen power” INCREASING BASICITY
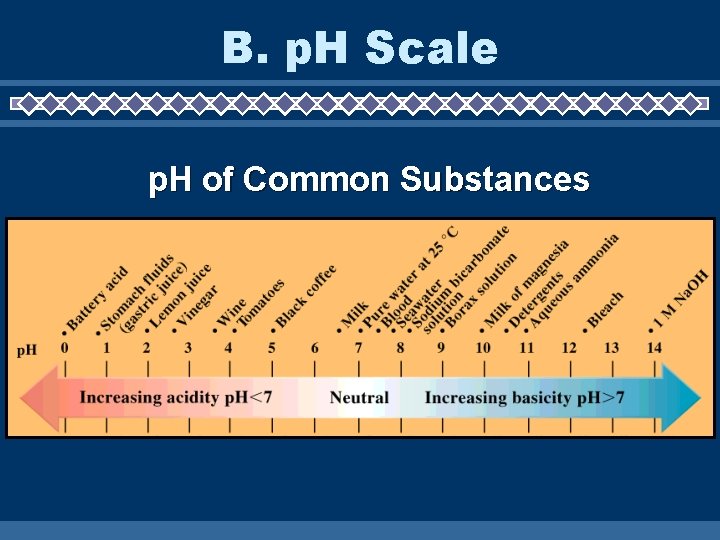
B. p. H Scale p. H of Common Substances
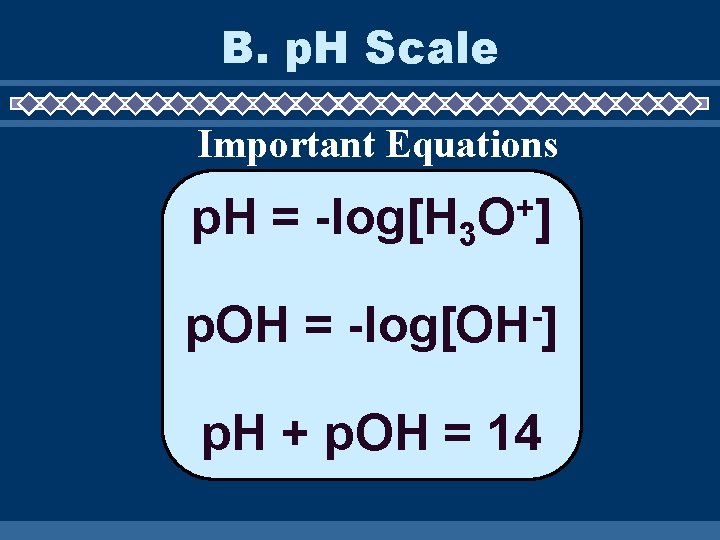
B. p. H Scale Important Equations p. H = -log[H 3 p. OH = + O] -log[OH ] p. H + p. OH = 14
![p. H = -log[H 3 p. OH = + O] -log[OH ] p. H p. H = -log[H 3 p. OH = + O] -log[OH ] p. H](http://slidetodoc.com/presentation_image_h2/fa35bab84d1177336bc1b36eff73b124/image-64.jpg)
p. H = -log[H 3 p. OH = + O] -log[OH ] p. H + p. OH = 14 [H 3 O+] = concentration of the acid [OH-] = concentratio n of the base

The p. H of sea water is about 7. 8. What is the p. OH? What do we know? p. H = 7. 8 What are we trying to figure out? p. OH = ? What equation should we use? p. H + p. OH = 14

The p. H of sea water is about 7. 8. What is the p. OH? p. H + p. OH = 14 7. 8 + p. OH = 14 Solve for p. OH = 14 – 7. 8 Calculate 6. 2
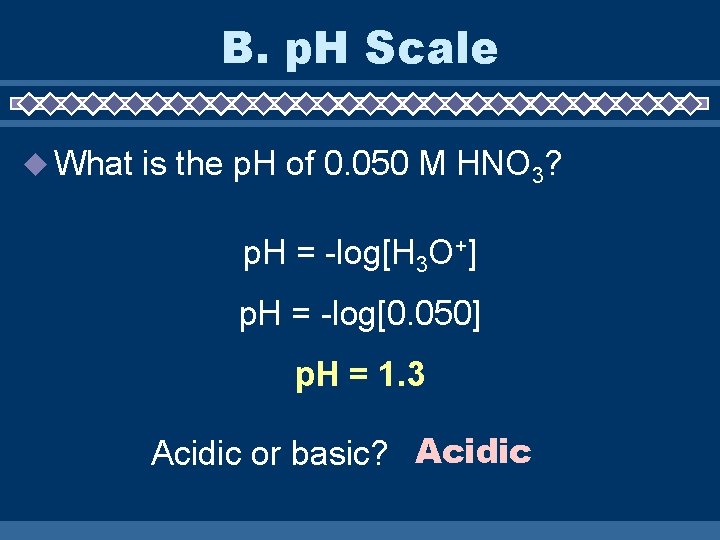
B. p. H Scale What is the p. H of 0. 050 M HNO 3? p. H = -log[H 3 O+] p. H = -log[0. 050] p. H = 1. 3 Acidic or basic? Acidic
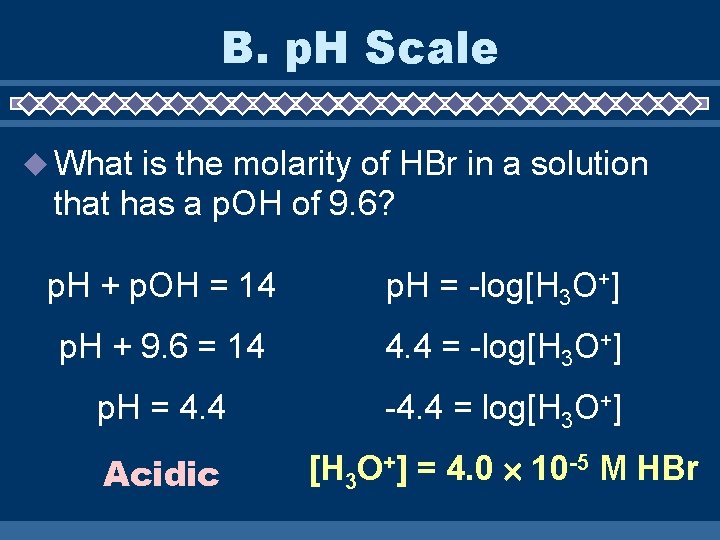
B. p. H Scale What is the molarity of HBr in a solution that has a p. OH of 9. 6? p. H + p. OH = 14 p. H = -log[H 3 O+] p. H + 9. 6 = 14 4. 4 = -log[H 3 O+] p. H = 4. 4 -4. 4 = log[H 3 O+] Acidic [H 3 O+] = 4. 0 10 -5 M HBr

Example Problems 1. 2. 3. 4. 5. What is the p. H of a substance that has a p. OH of 10. 3? 3. 7 What is the concentration of an acid that has the p. H of 4. 3? 5. 0 x 10 -5 M What is the concentration of a base that has the p. H of 8. 9? 7. 9 x 10 -6 M What is the p. H of an substance with the [H 3 O+] concentration of 5. 0 x 10 -5 M? 4. 3 What is the p. OH of a substance with the [H O+] concentration of 4. 2 x 10 -13? 1. 6
Titrations
A. Neutralization Chemical reaction between an acid and a base. Products are a salt (ionic compound) and water.
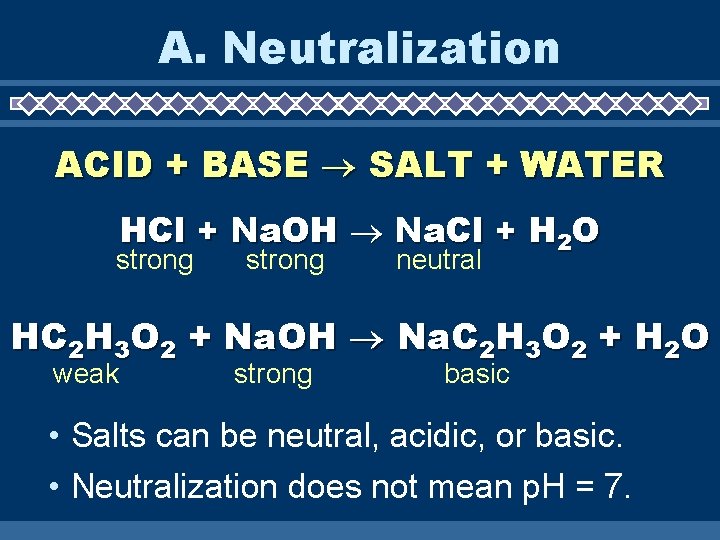
A. Neutralization ACID + BASE SALT + WATER HCl + Na. OH Na. Cl + H 2 O strong neutral HC 2 H 3 O 2 + Na. OH Na. C 2 H 3 O 2 + H 2 O weak strong basic • Salts can be neutral, acidic, or basic. • Neutralization does not mean p. H = 7.

B. Titration standard solution • Analytical method in which a standard solution is used to determine the concentration of an unknown solution
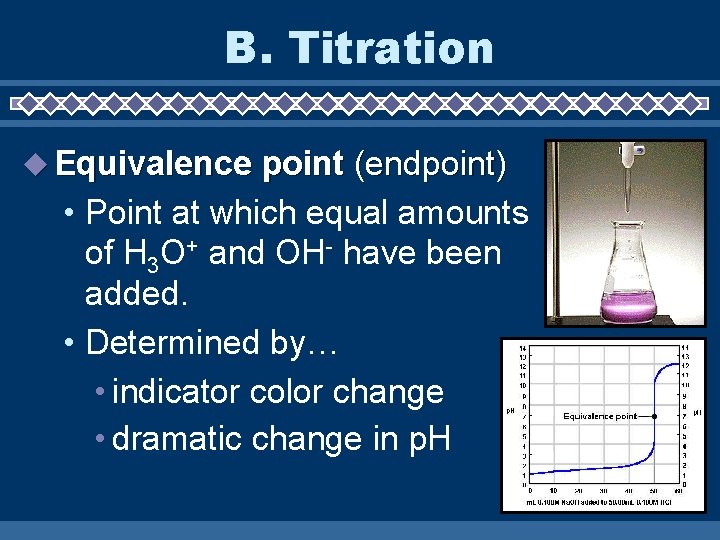
B. Titration Equivalence point (endpoint) • Point at which equal amounts of H 3 O+ and OH- have been added. • Determined by… • indicator color change • dramatic change in p. H
- Slides: 74