Solutions A solution is a homogenous mixture whose







- Slides: 17

Solutions ¡ ¡ A solution is a homogenous mixture whose component substances cannot be distinguished. Eg: Salt water, Gatorade, tap water, vodka, Kool-Aid.

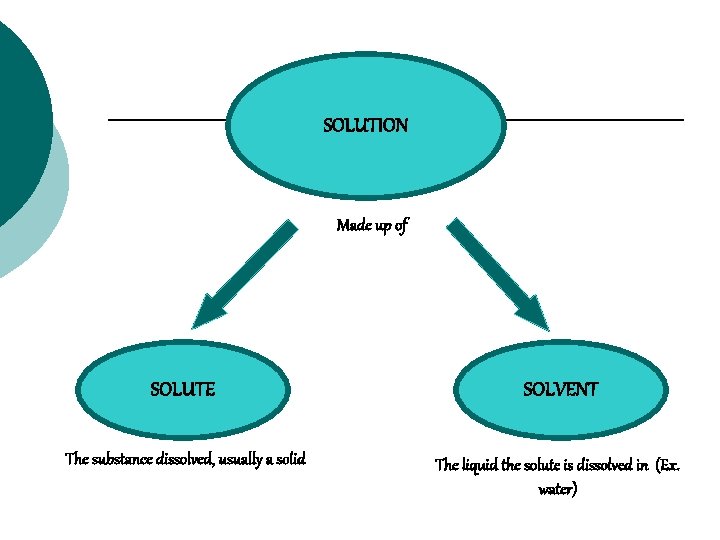
SOLUTION Made up of SOLUTE SOLVENT The substance dissolved, usually a solid The liquid the solute is dissolved in (Ex. water)

Orange Juice Solute: orange concentrate Solvent: watersdfsdf Common Solutions in the world…. . Salt Water Solute: salt Solvent: water Chocolate Milk Solute: chocolate powder Solvent: milk http: //www. nrprcd. org/photo_gallery/rcd_03/coordinator_breakfast/images/orange_juice. jpg http: //www. seedownunder. com. au/destination/queensland/noosa-accommodation/noosa-heads/ocean_breeze/images/OCEAN_BR

Aqueous Solution ¡ An aqueous solution is one in which the solvent is water

Solubility ¡ The maximum amount of solute that can be dissolved in a certain volume of solvent l l Solubility of solids increases with temperature (eg. Think of dissolving sugar in hot coffee) Solubility of gases decreases with temperature.

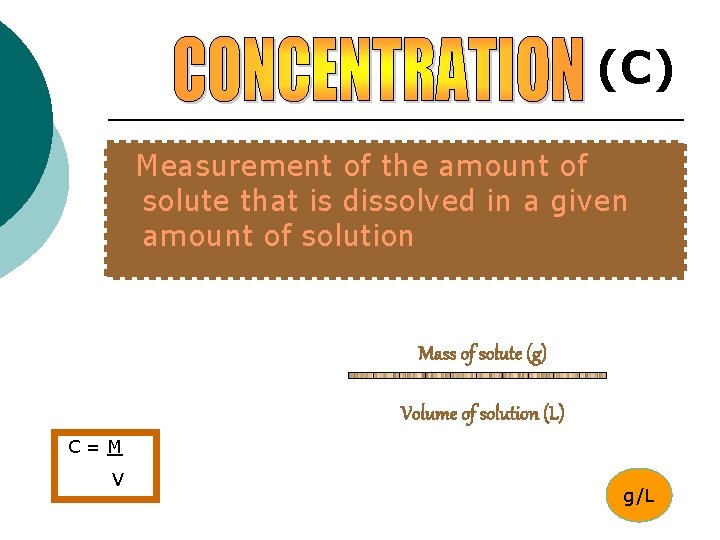
(C) Measurement of the amount of solute that is dissolved in a given amount of solution Mass of solute (g) Volume of solution (L) C=M V g/L

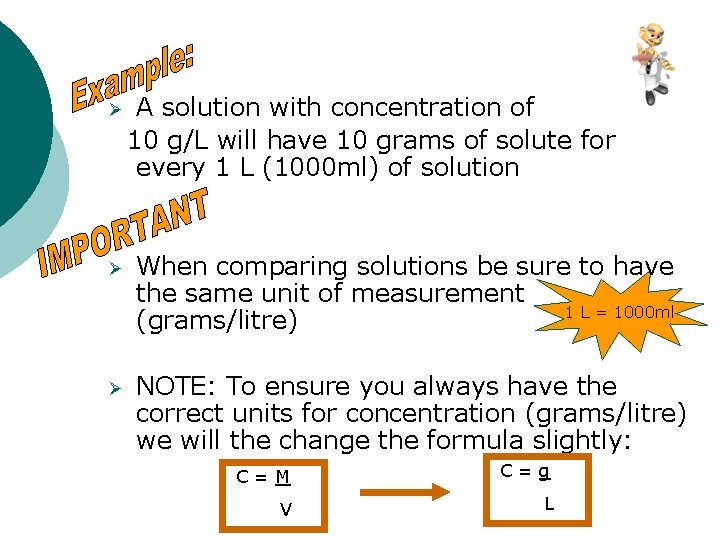
Ø A solution with concentration of 10 g/L will have 10 grams of solute for every 1 L (1000 ml) of solution Ø When comparing solutions be sure to have the same unit of measurement 1 L = 1000 ml (grams/litre) Ø NOTE: To ensure you always have the correct units for concentration (grams/litre) we will the change the formula slightly: C=M C=g V L

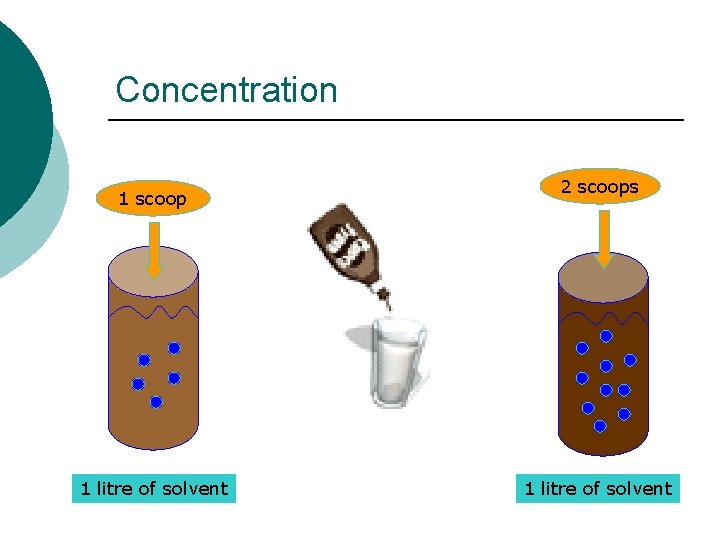
Concentration 1 scoop 1 litre of solvent 2 scoops 1 litre of solvent

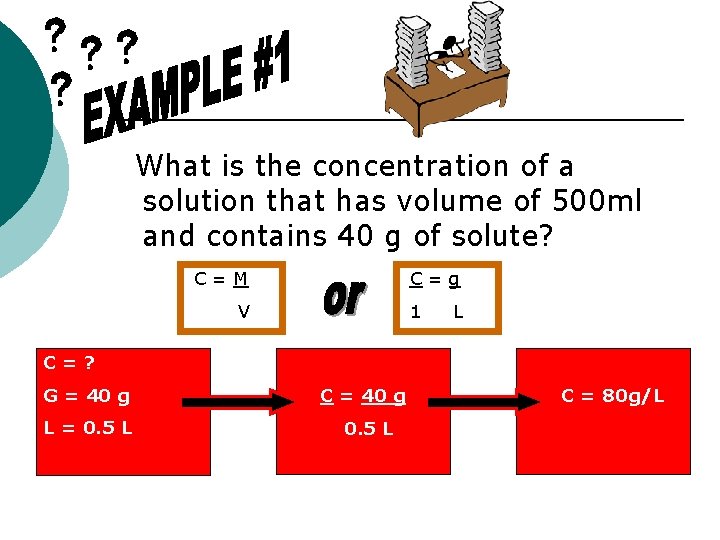
What is the concentration of a solution that has volume of 500 ml and contains 40 g of solute? C=M C=g V 1 L C=? G = 40 g L = 0. 5 L C = 40 g 0. 5 L C = 80 g/L

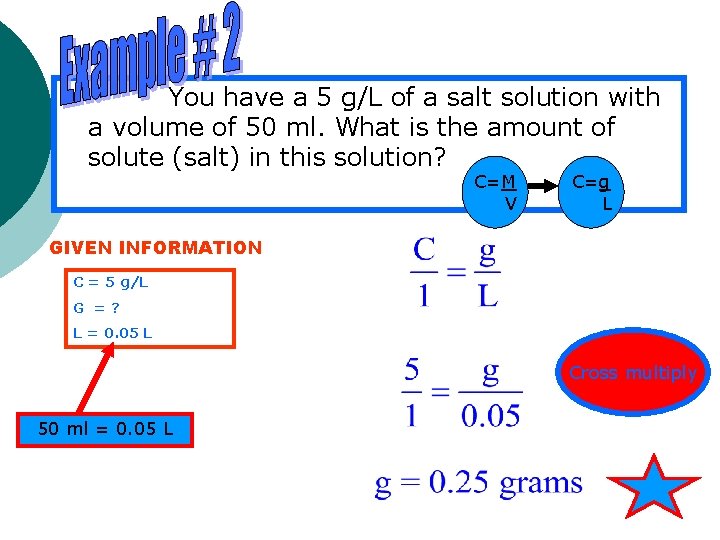
You have a 5 g/L of a salt solution with a volume of 50 ml. What is the amount of solute (salt) in this solution? C=M V C=g L GIVEN INFORMATION C = 5 g/L G =? L = 0. 05 L Cross multiply 50 ml = 0. 05 L

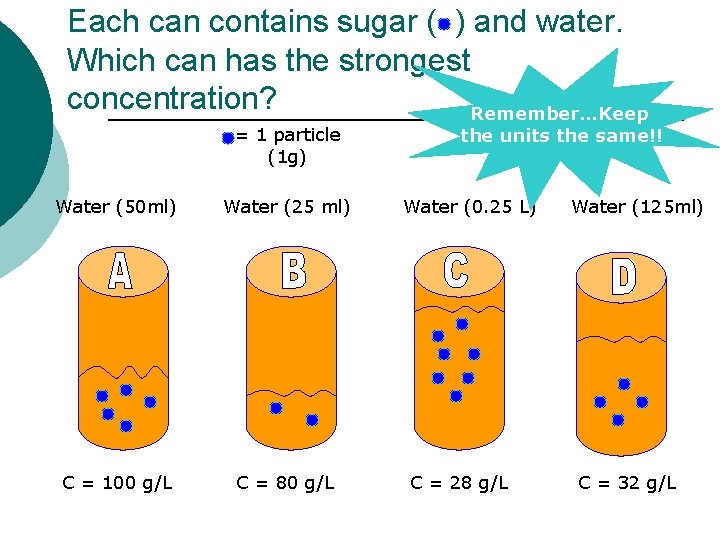
Each can contains sugar ( ) and water. Which can has the strongest concentration? Remember…Keep = 1 particle (1 g) Water (50 ml) Water (25 ml) C = 100 g/L C = 80 g/L the units the same!! Water (0. 25 L) C = 28 g/L Water (125 ml) C = 32 g/L

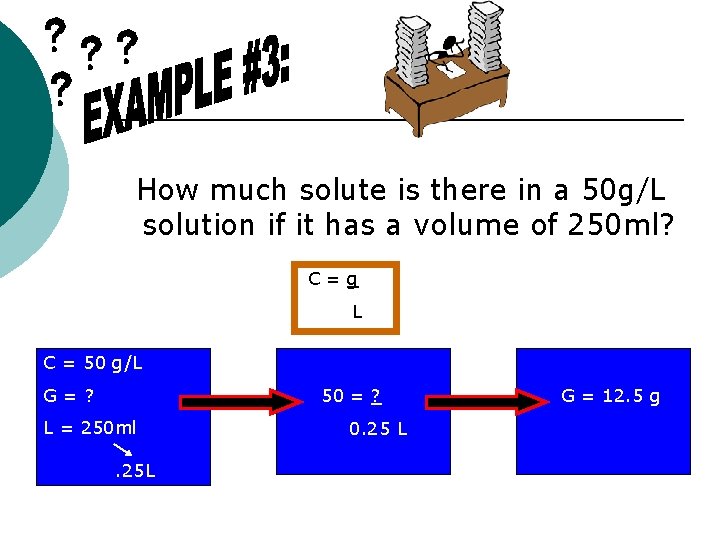
How much solute is there in a 50 g/L solution if it has a volume of 250 ml? C=g L C = 50 g/L G=? 50 = ? L = 250 ml. 25 L 0. 25 L G = 12. 5 g

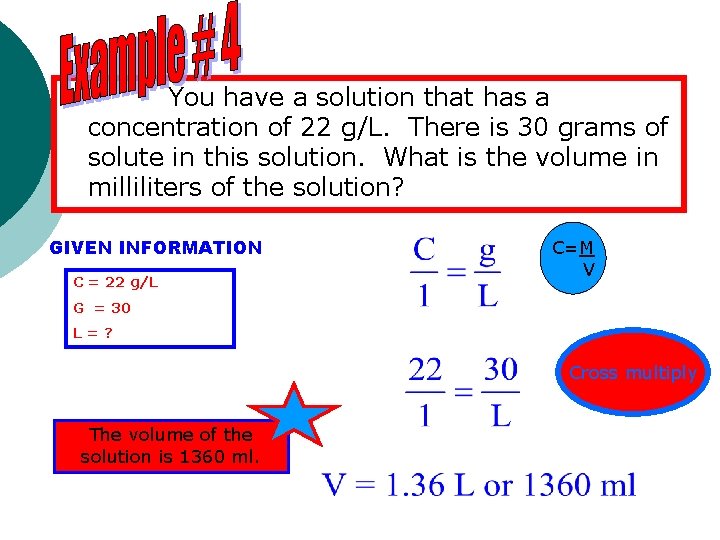
You have a solution that has a concentration of 22 g/L. There is 30 grams of solute in this solution. What is the volume in milliliters of the solution? GIVEN INFORMATION C = 22 g/L C=M V G = 30 L=? Cross multiply The volume of the solution is 1360 ml.

¡Place about half the amount of solvent (liquid) into container ¡Add the solute ¡Dissolve by shaking or swirling ¡Add the rest of the solvent (liquid) to reach the desired volume DO NOT put all of the solvent and solute together at once…inaccurate results!

Describe the procedure that would be used to prepare 250 ml of a sugar solution that has a concentration of 13 g/L. BE SPECIFIC…… C=g L Find out you how much grams you need. Write out procedure using a step by step method! 1. Pour half the required volume of solvent (125 ml) into a volumetric flask 2. Add the solute (3. 25 g of sugar) to the flask 3. Mix them together by swirlling. 4. Add more solvent (liquid) until you reach the desired final volume of 250 ml.

o Concentration Formula C =M/V (C=g/L) o To Prepare a Solution You…. -pour half of required amount of solvent in beaker -Add desired amount of solute -Mix -Add solvent (liquid) till your beaker reaches the required amount

Study guide, Module 3, page 14 ¡ Text book page 351 # 5, 6, 7 ¡ Worksheet # 4 ¡