Solutions 1 1 SOLUTION homogeneous mixture of two












- Slides: 30

Solutions 1

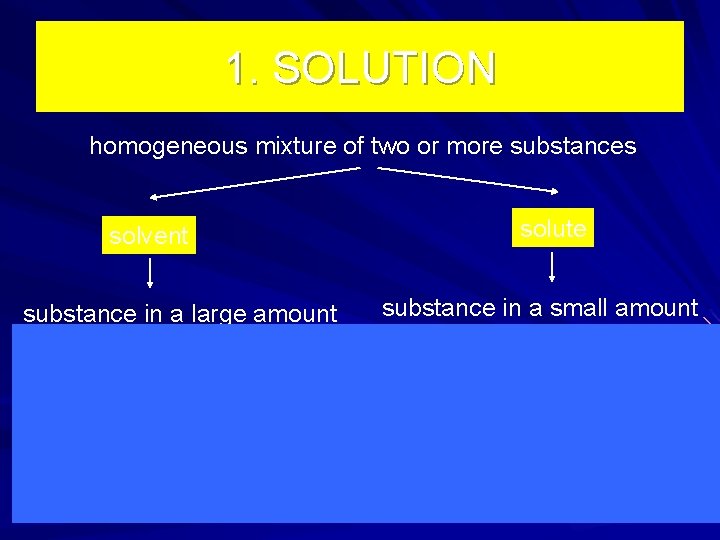
1. SOLUTION homogeneous mixture of two or more substances solute solvent substance in a large amount substance in a small amount N 2 gas phase (air) O 2 Ag solid phase (alloys) Au H 2 O liquid phase (sea water) Na. Cl 2

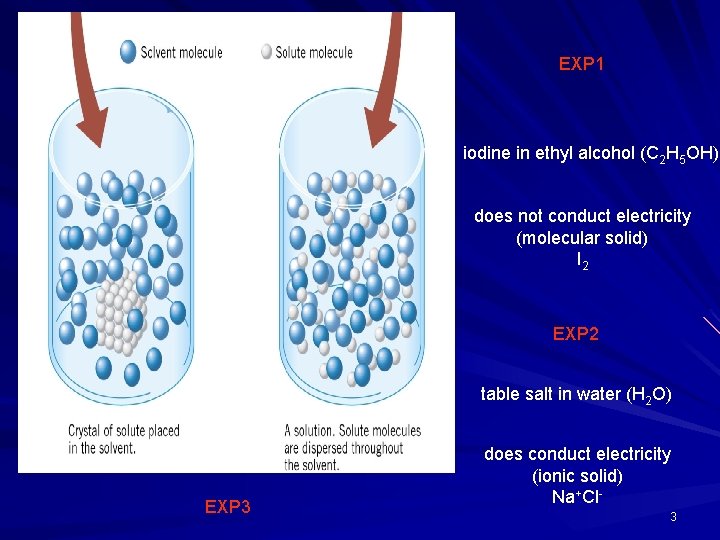
EXP 1 iodine in ethyl alcohol (C 2 H 5 OH) does not conduct electricity (molecular solid) I 2 EXP 2 table salt in water (H 2 O) EXP 3 does conduct electricity (ionic solid) Na+Cl 3

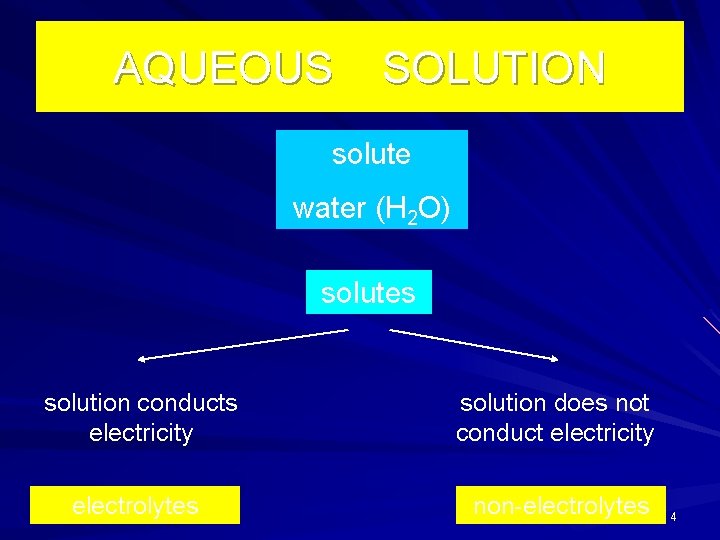
AQUEOUS SOLUTION solute water (H 2 O) solutes solution conducts electricity solution does not conduct electricity electrolytes non-electrolytes 4

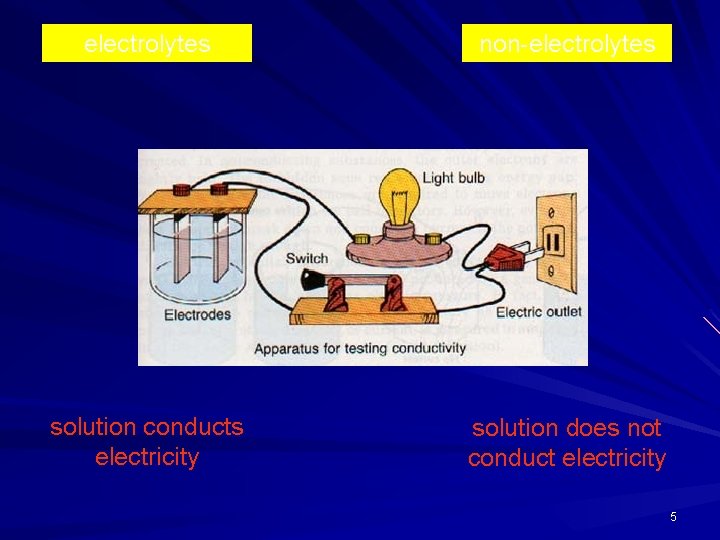
electrolytes non-electrolytes solution conducts electricity solution does not conduct electricity 5

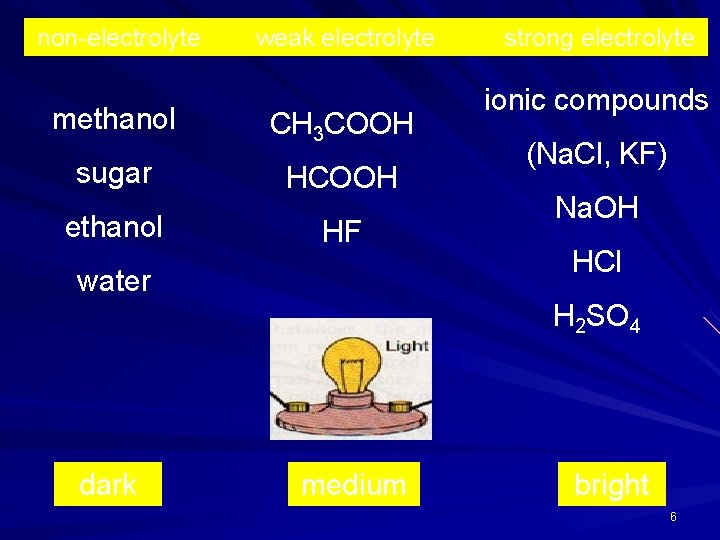
non-electrolyte methanol sugar ethanol weak electrolyte CH 3 COOH HF water strong electrolyte ionic compounds (Na. Cl, KF) Na. OH HCl H 2 SO 4 dark medium bright 6

SOLUTION EXP 4 concentration 7

GAS PHASE SOLUTION Saturn solvent H 2/He solute CH 4, PH 3 8

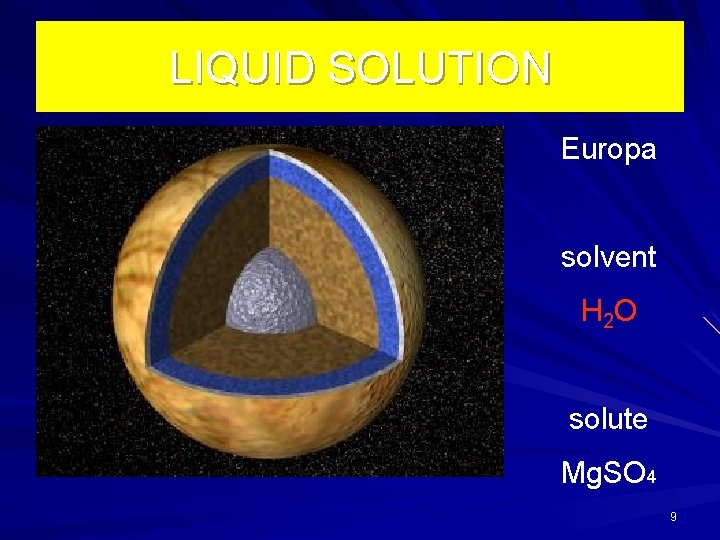
LIQUID SOLUTION Europa solvent H 2 O solute Mg. SO 4 9

SOLID SOLUTION Triton solvent N 2 solute CH 4 10

Components of Solutions Component: a substance that is part of the solution Solvent: present in the greatest amount Solute: other than solvent (lower amount) 11

Mixtures that are not Solutions Colloids – appears to be a homogeneous mixture, but particles are much bigger, but not filterable. E. g. Fog, smoke, whipped cream, mayonnaise, etc. Suspension: larger particle sizes, filterable. E. g. mud, freshly squeezed orange juice. 12

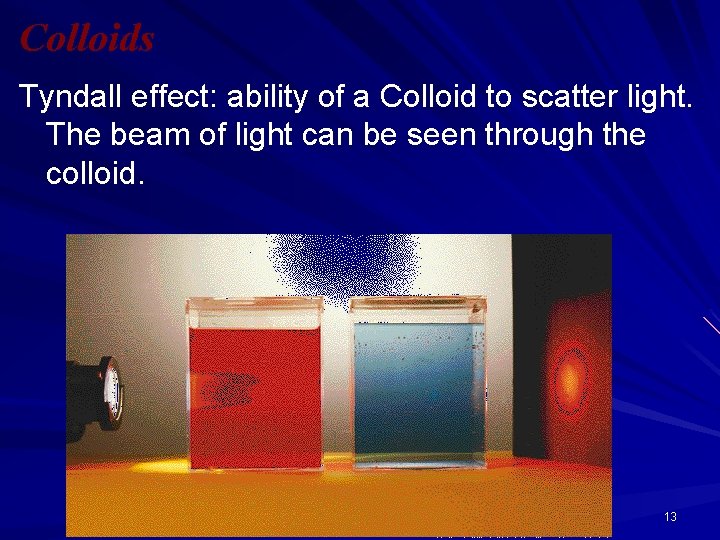
Colloids Tyndall effect: ability of a Colloid to scatter light. The beam of light can be seen through the colloid. 13

Solubility and Solutions Solubility: Amount of solute that dissolves in a solvent to produce a saturated solution. (Solubility often expressed in g/100 m. L. ) E. g. 0. 30 g of I 2 dissolved in 1000 g of H 2 O. Saturated solution: maximum amount of solute is dissolved in solvent. Trying to dissolve more results in undissolved solute in container. 14

Unsaturated solution: less than max. amount of solute is dissolved in solvent. Supersaturation = more solute in solution than normally allowed; we call this a supersaturated solution. 15

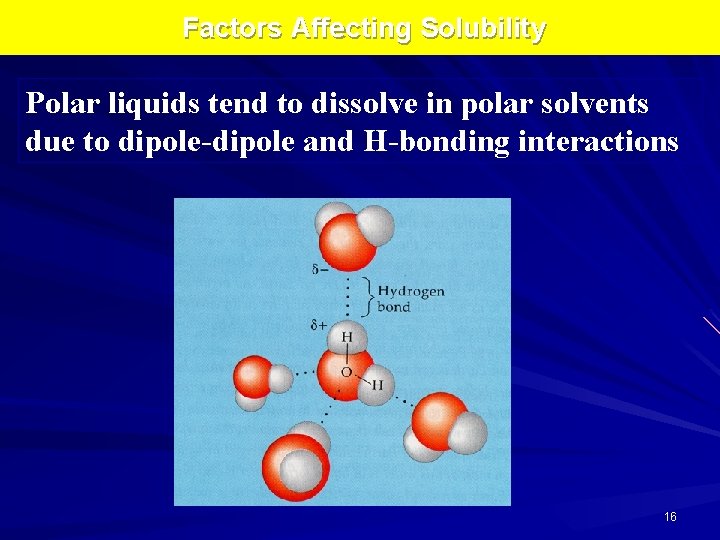
Factors Affecting Solubility Polar liquids tend to dissolve in polar solvents due to dipole-dipole and H-bonding interactions 16

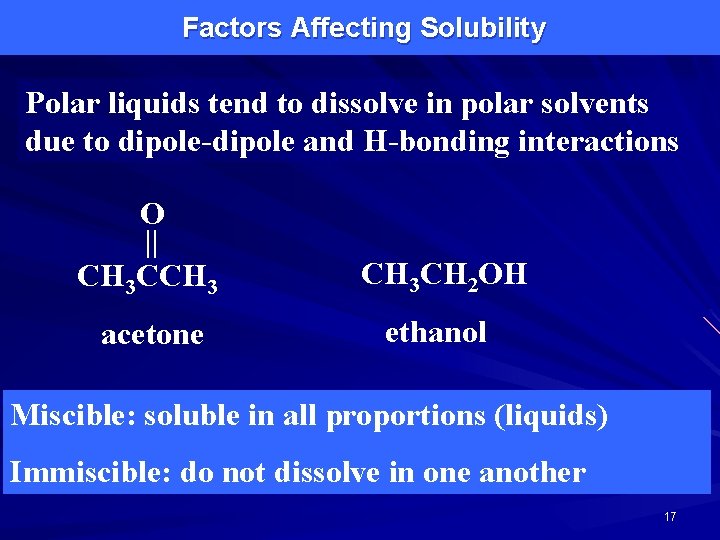
Factors Affecting Solubility Polar liquids tend to dissolve in polar solvents due to dipole-dipole and H-bonding interactions O || CH 3 CCH 3 CH 2 OH acetone ethanol Miscible: soluble in all proportions (liquids) Immiscible: do not dissolve in one another 17

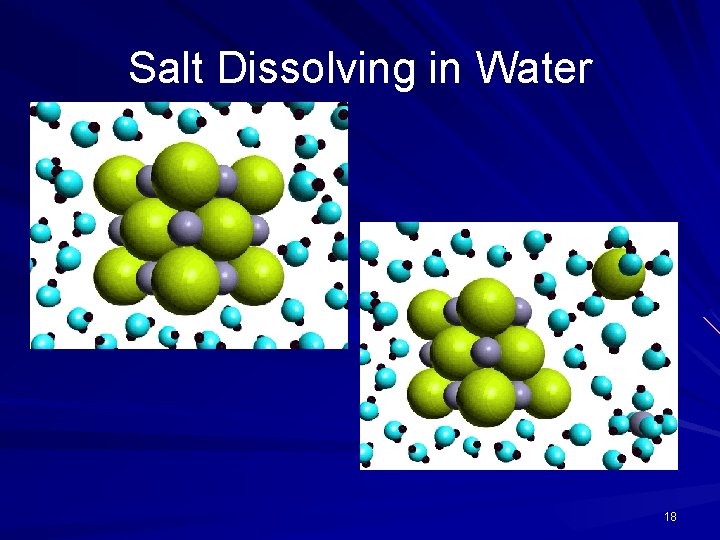
Salt Dissolving in Water 18

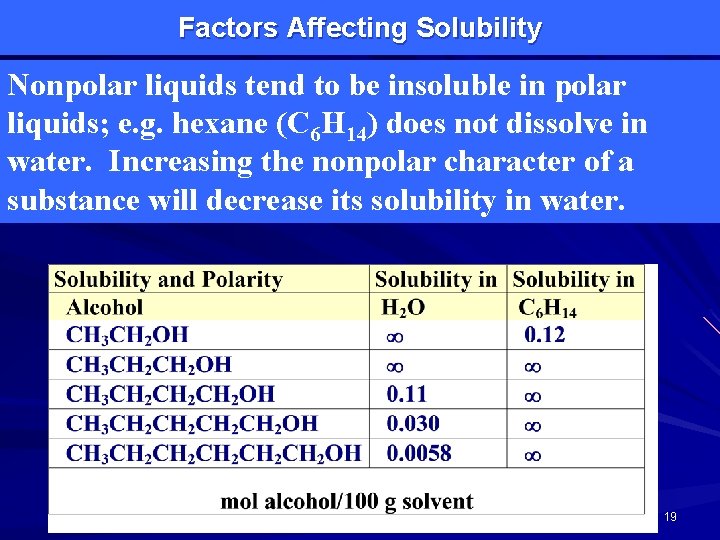
Factors Affecting Solubility Nonpolar liquids tend to be insoluble in polar liquids; e. g. hexane (C 6 H 14) does not dissolve in water. Increasing the nonpolar character of a substance will decrease its solubility in water. 19

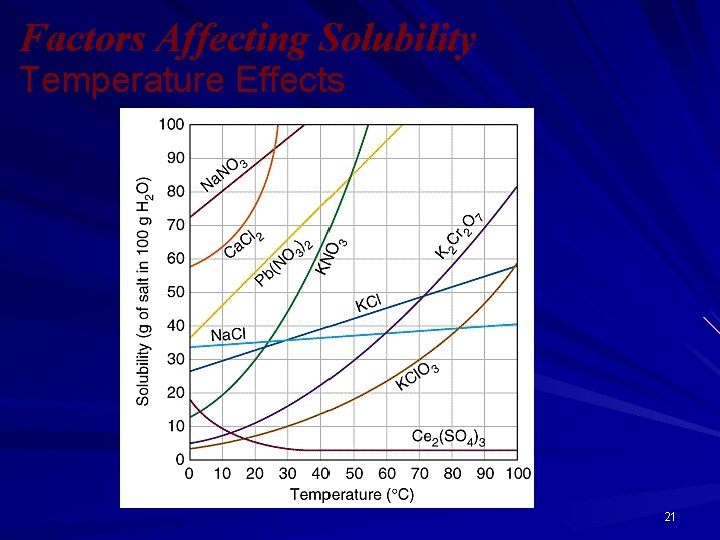
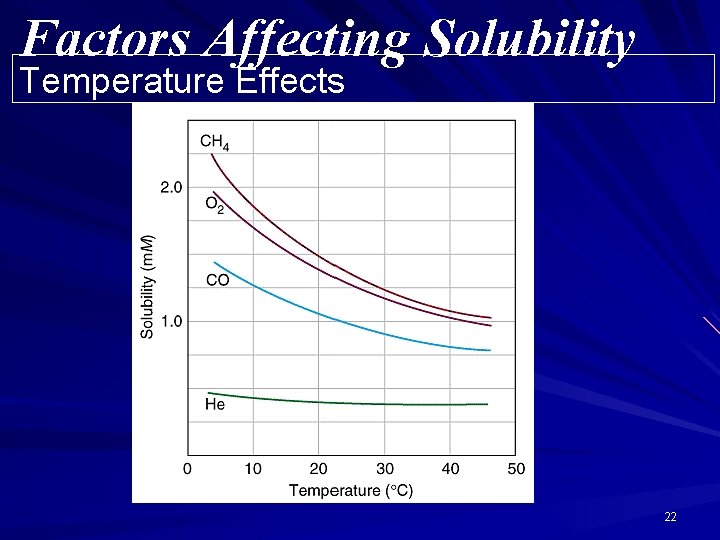
Solubility: Temperature Dependence All solubilities are temperature dependent; Most solids are more soluble at higher temperatures. All gases are less soluble at higher temperatures. 20

Factors Affecting Solubility Temperature Effects 21

Factors Affecting Solubility Temperature Effects 22

Ways of Expressing Concentration: intensive property that conveys the amount of solute relative to the amount of either solvent or solution Qualitative ways: Dilute Concentrated Quantitative ways: Numeric representation Several ways 23

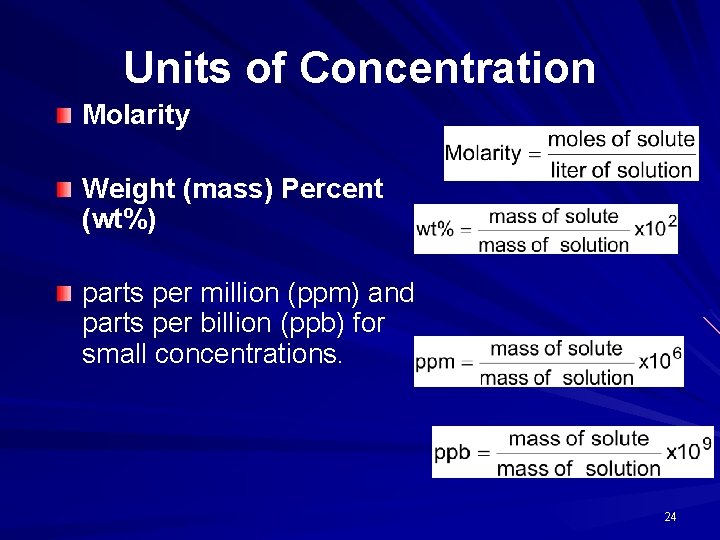
Units of Concentration Molarity Weight (mass) Percent (wt%) parts per million (ppm) and parts per billion (ppb) for small concentrations. 24

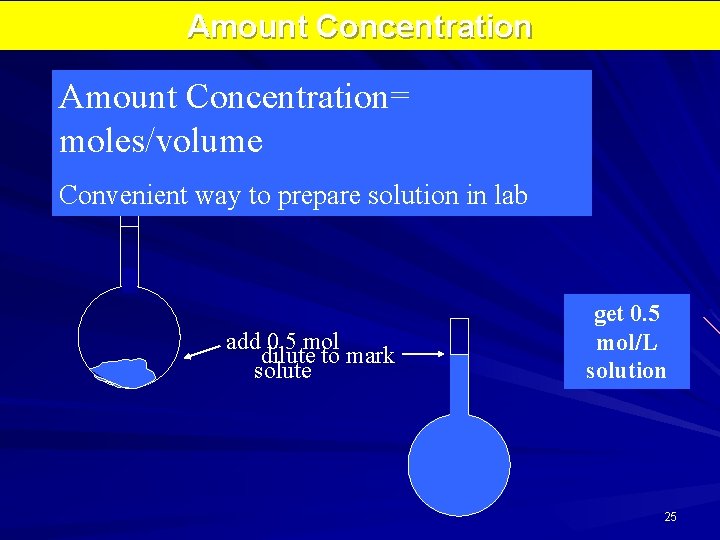
Amount Concentration= moles/volume 1 -L flask (1000. 0 m. L) Convenient way to prepare solution in lab add 0. 5 mol dilute to mark solute get 0. 5 mol/L solution 25

Example How many grams of sulfur Hydride (H 2 S) must be dissolved in water to make 1 L of a 0. 12 mol/L Solution 26

Example How many grams of sulfur Hydride (H 2 S) must be dissolved in 1 L of water to make a 0. 12 mol/L solution? 0. 12 mol x (34. 09 g/1 mol) = 4. 09 g= 4. 1 Dissolve in enough water to make 1 litre 27

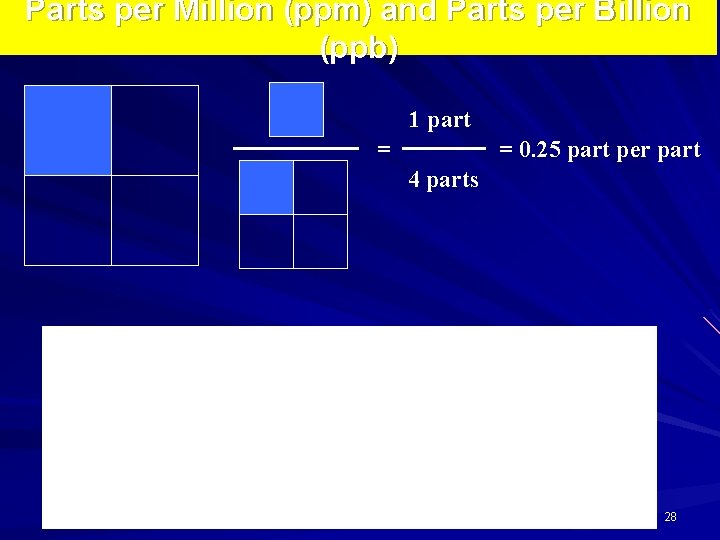
Parts per Million (ppm) and Parts per Billion (ppb) 1 part = = 0. 25 part per part 4 parts 28

Mass percentage e. g. Solution of HCl that is 36% by mass contains 36 g of HCl for every 100 g solution 29

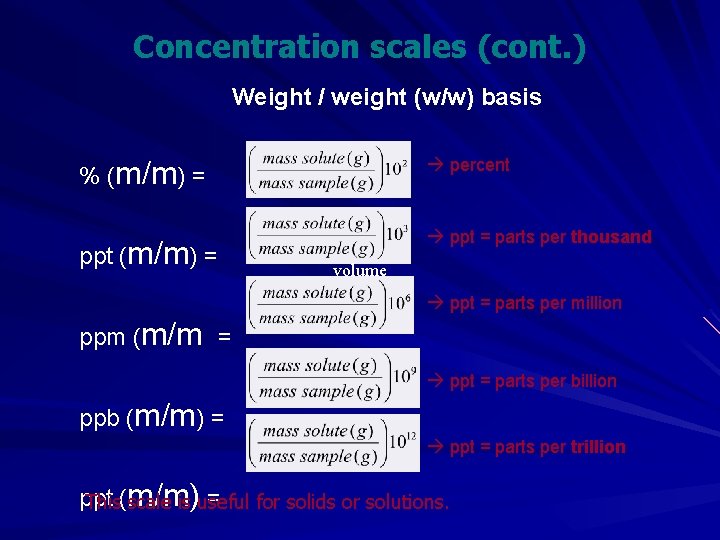
Concentration scales (cont. ) Weight / weight (w/w) basis % (m/m) = percent ppt (m/m) = ppt = parts per thousand volume ppt = parts per million ppm (m/m = ppt = parts per billion ppb (m/m) = ppt = parts per trillion ppt = for solids or solutions. This(m/m) scale is useful