SOLUTION S UNIT Introduction Net Ionic Equations What





- Slides: 9

SOLUTION S UNIT Introduction & Net Ionic Equations

What is a solution? Solution: A homogeneous mixture – The components are equally spread out – A sample from one part is the same as any other – (The first sip of your 7 -up is the same as the last!)

Components of a Solution: – Solvent: The substance that is present in the largest amount – Solute: The substance that is present in the smallest amount – Example—when you dissolve a tsp of salt in a glass of water: • Solvent = water • Solute = salt

Aqueous Solutions • In an aqueous solution—water is the solvent – This is the type of solution we will be focusing on! • Saltwater is an aqueous solution. • Water is a good solvent because it is a polar molecule! – Review: What makes water a polar molecule? ? ?

How does a solution form? (for ionic substances) Ionic substances have two forces acting on them: 1. The attraction of the ions that make up the substance to each other (ex: Na+ & Cl-) 2. The attraction of the ions to the positive and negative ends of the water molecules Ionic substances dissolve when the attractions of the ions to the water molecules are stronger than their attractions to each other http: //www. northland. cc. mn. us/biology/BIOLOGY 1111/animations/dissolve. html

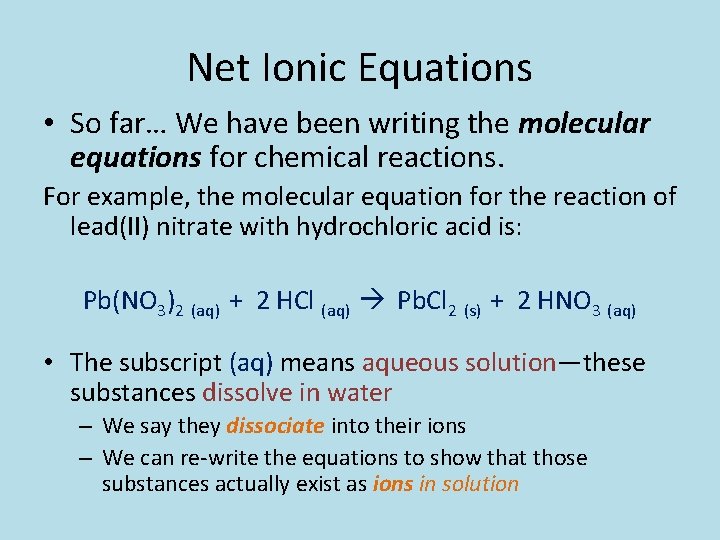
Net Ionic Equations • So far… We have been writing the molecular equations for chemical reactions. For example, the molecular equation for the reaction of lead(II) nitrate with hydrochloric acid is: Pb(NO 3)2 (aq) + 2 HCl (aq) Pb. Cl 2 (s) + 2 HNO 3 (aq) • The subscript (aq) means aqueous solution—these substances dissolve in water – We say they dissociate into their ions – We can re-write the equations to show that those substances actually exist as ions in solution

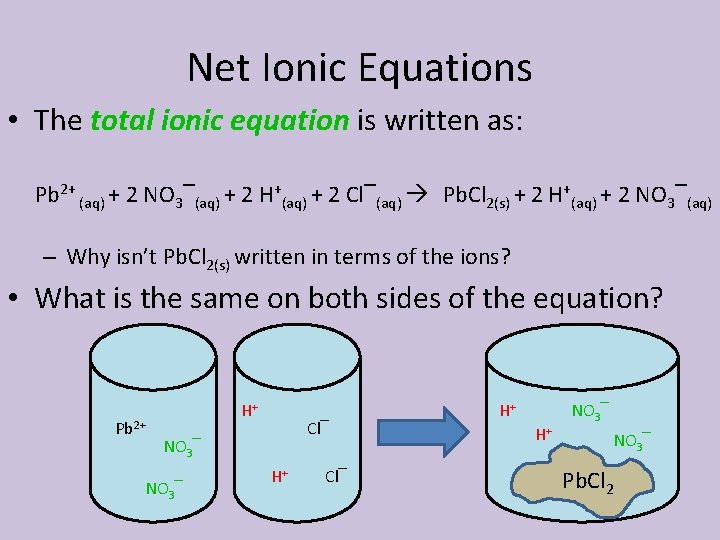
Net Ionic Equations • The total ionic equation is written as: Pb 2+ (aq) + 2 NO 3‾(aq) + 2 H+(aq) + 2 Cl‾(aq) Pb. Cl 2(s) + 2 H+(aq) + 2 NO 3‾(aq) – Why isn’t Pb. Cl 2(s) written in terms of the ions? • What is the same on both sides of the equation? Pb 2+ H+ Cl‾ NO 3‾ H+ Cl‾ H+ H+ NO 3‾ Pb. Cl 2

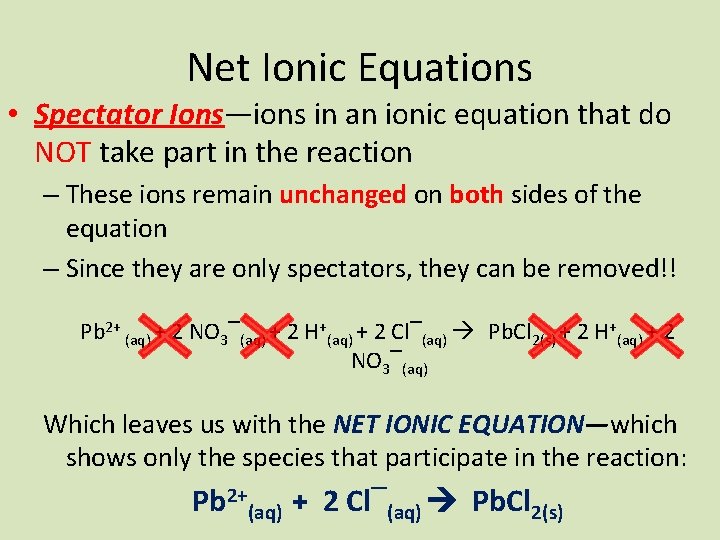
Net Ionic Equations • Spectator Ions—ions in an ionic equation that do NOT take part in the reaction – These ions remain unchanged on both sides of the equation – Since they are only spectators, they can be removed!! Pb 2+ (aq) + 2 NO 3‾(aq) + 2 H+(aq) + 2 Cl‾(aq) Pb. Cl 2(s) + 2 H+(aq) + 2 NO 3‾(aq) Which leaves us with the NET IONIC EQUATION—which shows only the species that participate in the reaction: Pb 2+(aq) + 2 Cl‾(aq) Pb. Cl 2(s)

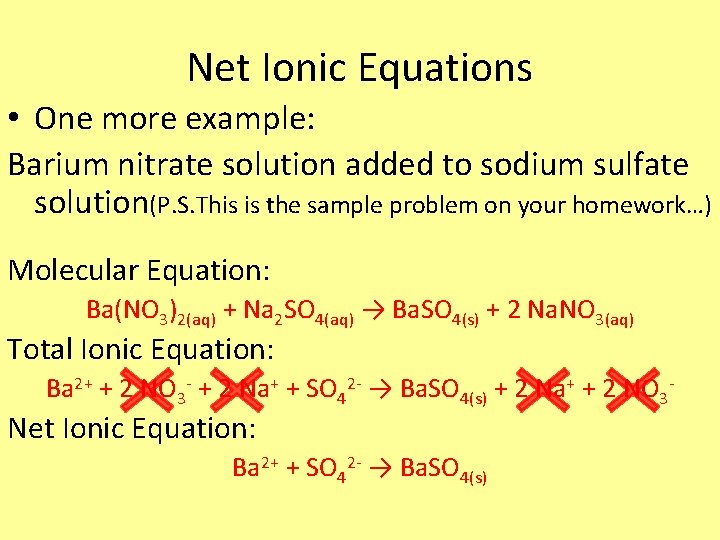
Net Ionic Equations • One more example: Barium nitrate solution added to sodium sulfate solution(P. S. This is the sample problem on your homework…) Molecular Equation: Ba(NO 3)2(aq) + Na 2 SO 4(aq) → Ba. SO 4(s) + 2 Na. NO 3(aq) Total Ionic Equation: Ba 2+ + 2 NO 3 - + 2 Na+ + SO 42 - → Ba. SO 4(s) + 2 Na+ + 2 NO 3 - Net Ionic Equation: Ba 2+ + SO 42 - → Ba. SO 4(s)