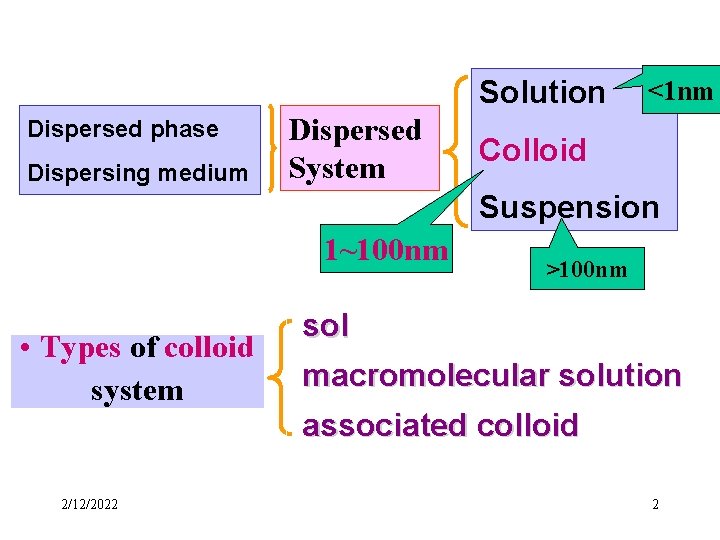
Solution Dispersed phase Dispersing medium Dispersed System 1




![The structure of Fe(OH)3 sol {[ Fe(OH)3]m·n. Fe. O+ ·(n-x) Cl- }x+ · x The structure of Fe(OH)3 sol {[ Fe(OH)3]m·n. Fe. O+ ·(n-x) Cl- }x+ · x](https://slidetodoc.com/presentation_image_h2/b095228be7c127487125cfaf15e96479/image-16.jpg)







- Slides: 41


Solution Dispersed phase Dispersing medium Dispersed System <1 nm Colloid Suspension 1~100 nm • Types of colloid system >100 nm sol macromolecular solution associated colloid 2/12/2022 2

4. 5. 1溶胶 Sol 1. Formation of Colloidal Particles • Dispersion methods: methods in which large pieces of the substance are broken up into particles of colloidal size. Ag sol preparation by the electric arc method. 2/12/2022 3

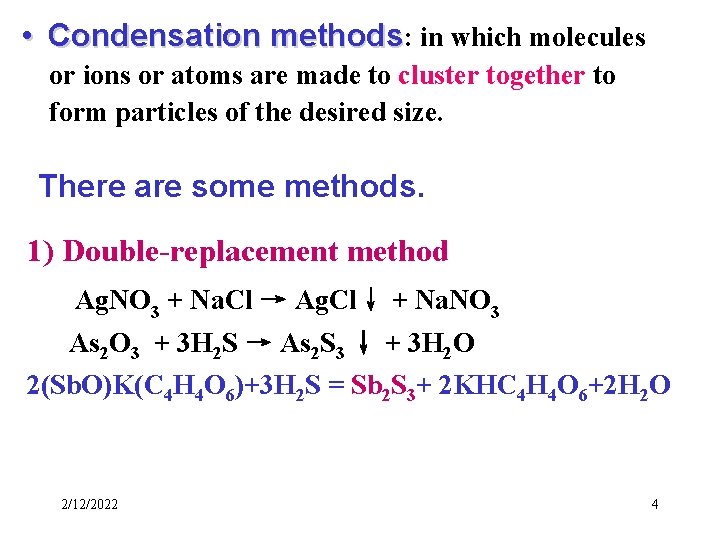
• Condensation methods: in which molecules or ions or atoms are made to cluster together to form particles of the desired size. There are some methods. 1) Double-replacement method Ag. NO 3 + Na. Cl → Ag. Cl↓ + Na. NO 3 As 2 O 3 + 3 H 2 S → As 2 S 3 ↓ + 3 H 2 O 2(Sb. O)K(C 4 H 4 O 6)+3 H 2 S = Sb 2 S 3+ 2 KHC 4 H 4 O 6+2 H 2 O 2/12/2022 4

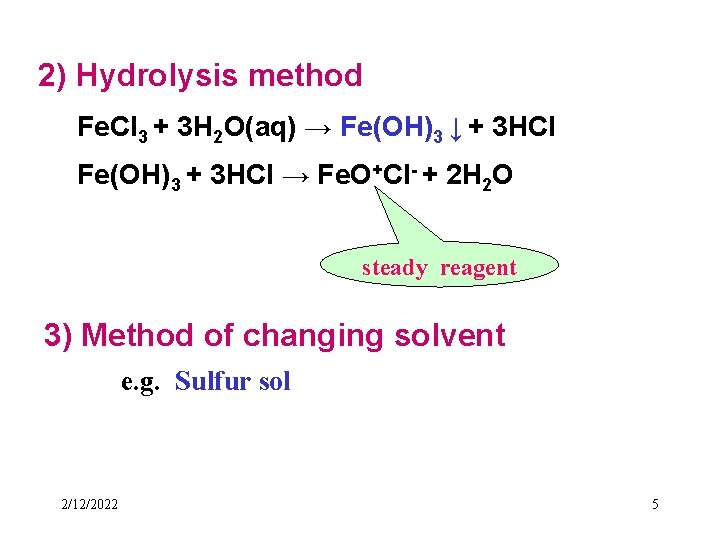
2) Hydrolysis method Fe. Cl 3 + 3 H 2 O(aq) → Fe(OH)3 ↓ + 3 HCl Fe(OH)3 + 3 HCl → Fe. O+Cl- + 2 H 2 O steady reagent 3) Method of changing solvent e. g. Sulfur sol 2/12/2022 5

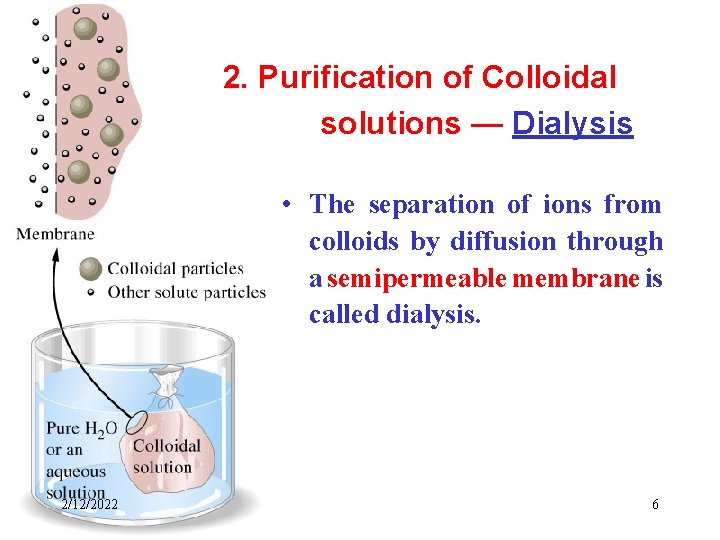
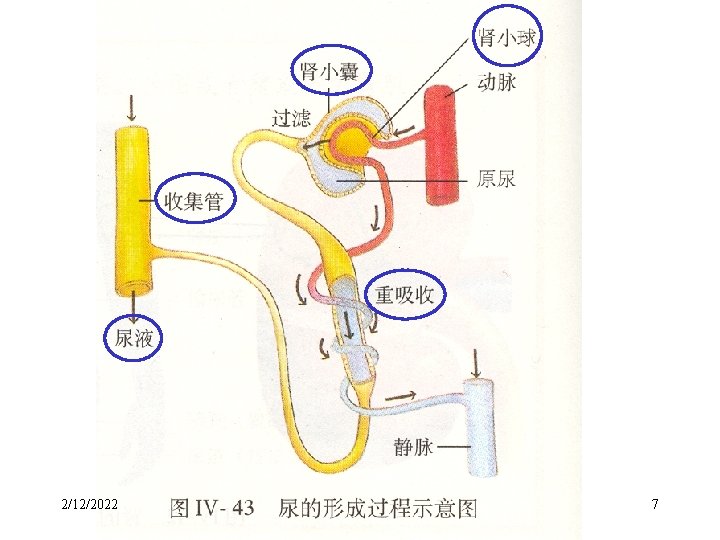
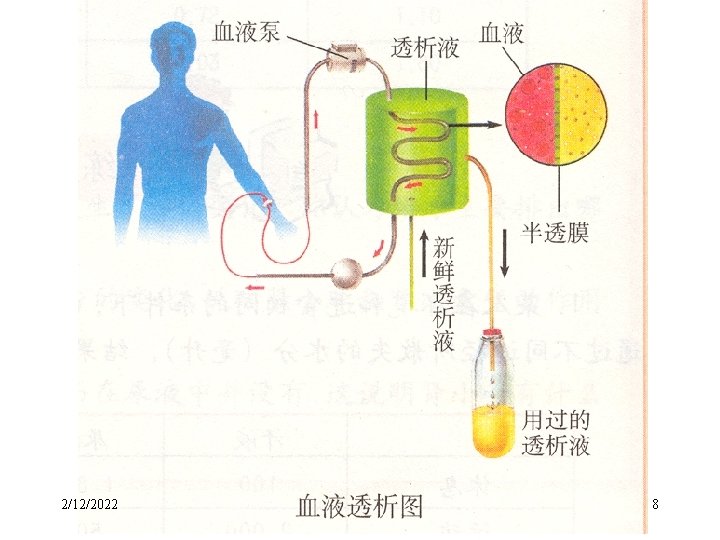
2. Purification of Colloidal solutions — Dialysis • The separation of ions from colloids by diffusion through a semipermeable membrane is called dialysis. 2/12/2022 6

2/12/2022 7

2/12/2022 8

3. Properties of Colloidal Dispersions • Optical behaviors of sol: Tyndall Effect • Electric behaviors of sol: Electrophoresis • Coagulation of Colloidal Dispersions 2/12/2022 9

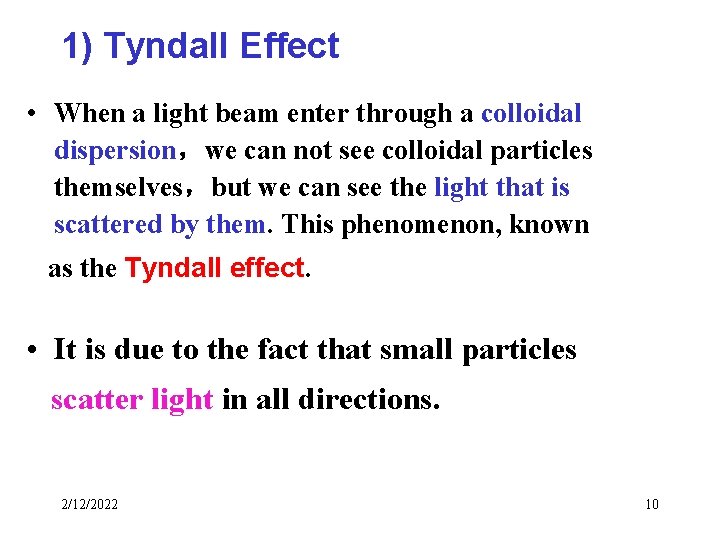
1) Tyndall Effect • When a light beam enter through a colloidal dispersion,we can not see colloidal particles themselves,but we can see the light that is scattered by them. This phenomenon, known as the Tyndall effect. • It is due to the fact that small particles scatter light in all directions. 2/12/2022 10

2/12/2022 11

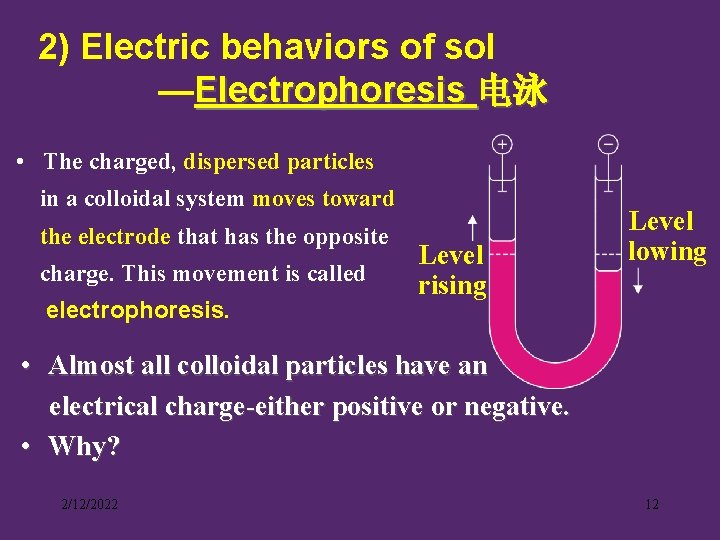
2) Electric behaviors of sol —Electrophoresis 电泳 • The charged, dispersed particles in a colloidal system moves toward the electrode that has the opposite charge. This movement is called electrophoresis. Level rising Level lowing • Almost all colloidal particles have an electrical charge-either positive or negative. • Why? 2/12/2022 12

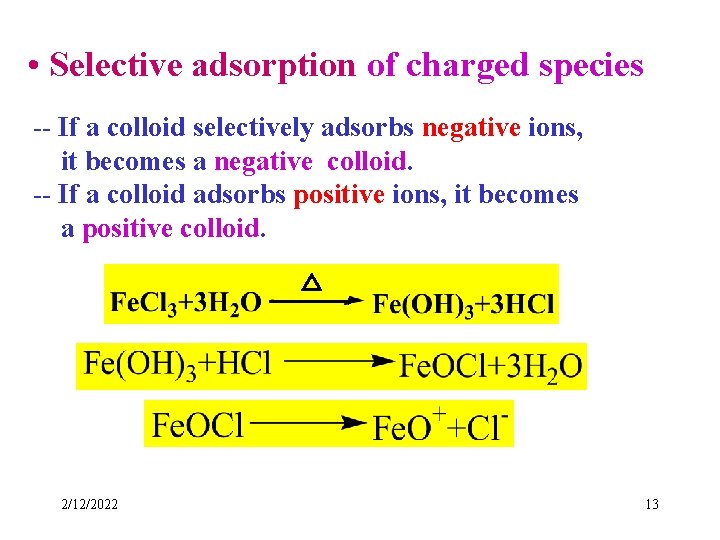
• Selective adsorption of charged species -- If a colloid selectively adsorbs negative ions, it becomes a negative colloid. -- If a colloid adsorbs positive ions, it becomes a positive colloid. △ 2/12/2022 13

Ag. NO 3 +KI → Ag. I↓+ KNO 3 If a dilute solution of a silver salt is added to a slight excess of dilute potassium iodide solution, a negatively charged sol of silver iodide is obtained; However, if the dilute iodide solution is added to excess of the silver nitrate, a positively charged sol results. If the two solution are mixed in exactly equivalent amounts, the silver iodide sol is unstable, and complete precipitation occurs. 2/12/2022 14

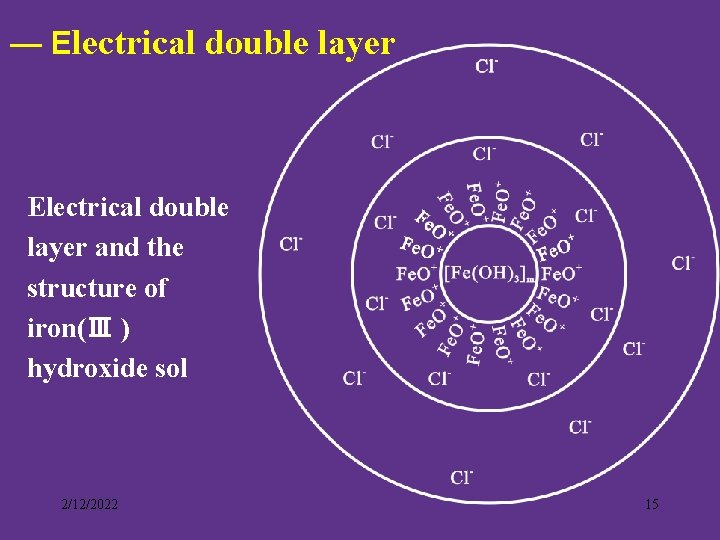
— Electrical double layer and the structure of iron(Ⅲ ) hydroxide sol 2/12/2022 15
![The structure of FeOH3 sol FeOH3mn Fe O nx Cl x x The structure of Fe(OH)3 sol {[ Fe(OH)3]m·n. Fe. O+ ·(n-x) Cl- }x+ · x](https://slidetodoc.com/presentation_image_h2/b095228be7c127487125cfaf15e96479/image-16.jpg)
The structure of Fe(OH)3 sol {[ Fe(OH)3]m·n. Fe. O+ ·(n-x) Cl- }x+ · x Cl吸附层 胶核 扩散层 胶粒 胶团 2/12/2022 16

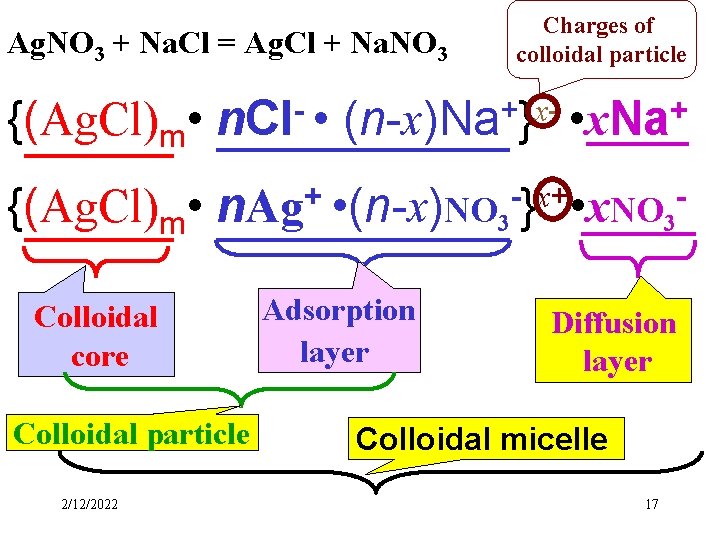
Charges of colloidal particle Ag. NO 3 + Na. Cl = Ag. Cl + Na. NO 3 {(Ag. Cl)m • n. Cl- • (n-x)Na+}x- • x. Na+ {(Ag. Cl)m • + x+ n. Ag • (n-x)NO } • x. NO Colloidal core Colloidal particle 2/12/2022 3 Adsorption layer 3 Diffusion layer Colloidal micelle 17

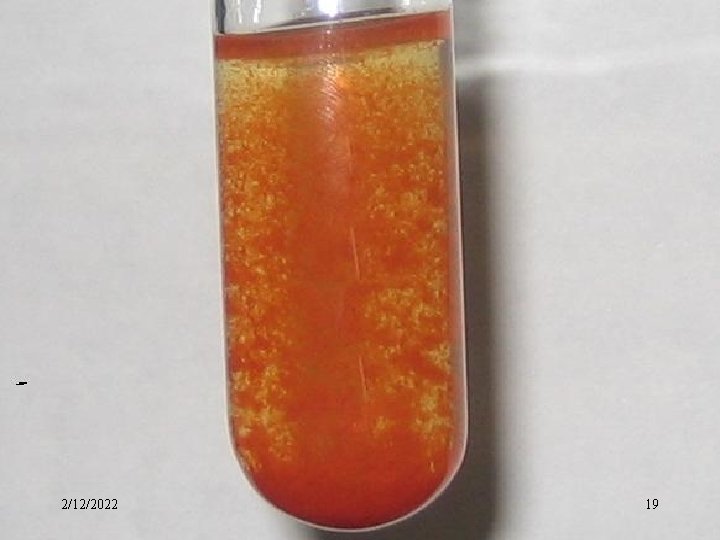
3)Coagulation of Colloidal Dispersions Coagulation is the process by which the dispersed phase of a colloid is made to aggregate and thereby separate from the dispersion medium. • The most effective way of coagulating colloidal dispersions of the sol is by adding an electrolyte. First, the ion which is effective in causing precipitation of a sol is the one whose charge is of opposite sign to that of the colloidal particles; Second, the precipitation effect increase markedly with increasing valence of the ion. 2/12/2022 18

2/12/2022 19

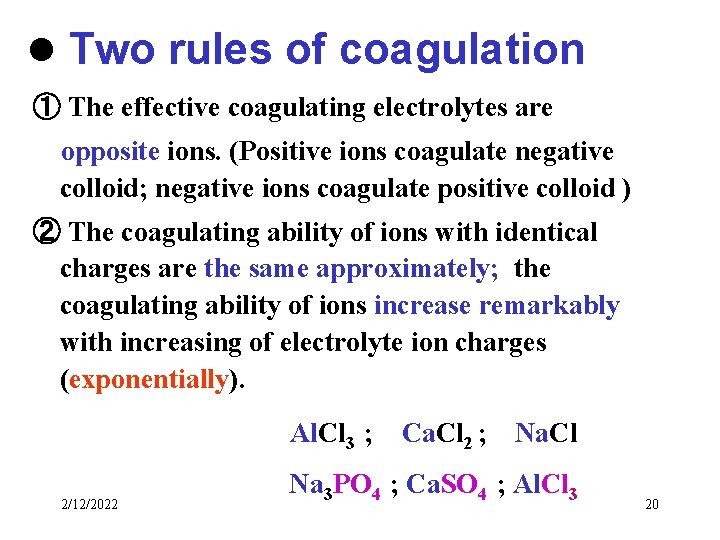
l Two rules of coagulation ① The effective coagulating electrolytes are opposite ions. (Positive ions coagulate negative colloid; negative ions coagulate positive colloid ) ② The coagulating ability of ions with identical charges are the same approximately; the coagulating ability of ions increase remarkably with increasing of electrolyte ion charges (exponentially). Al. Cl 3 ; 2/12/2022 Ca. Cl 2 ; Na. Cl Na 3 PO 4 ; Ca. SO 4 ; Al. Cl 3 20



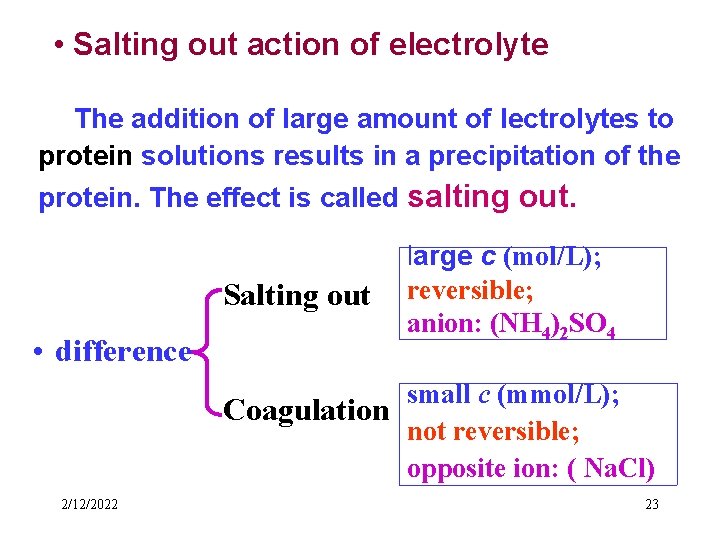
• Salting out action of electrolyte The addition of large amount of lectrolytes to protein solutions results in a precipitation of the protein. The effect is called salting out. Salting out • difference large c (mol/L); reversible; anion: (NH 4)2 SO 4 small c (mmol/L); Coagulation not reversible; opposite ion: ( Na. Cl) 2/12/2022 23

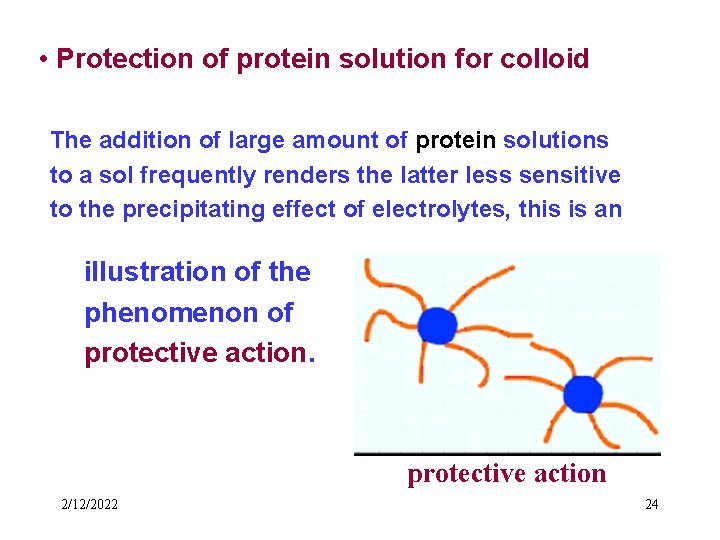
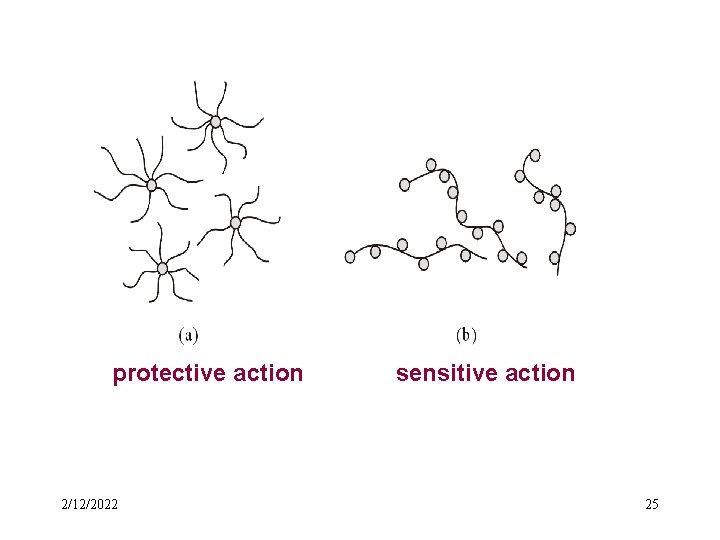
• Protection of protein solution for colloid The addition of large amount of protein solutions to a sol frequently renders the latter less sensitive to the precipitating effect of electrolytes, this is an illustration of the phenomenon of protective action 2/12/2022 24

protective action 2/12/2022 sensitive action 25

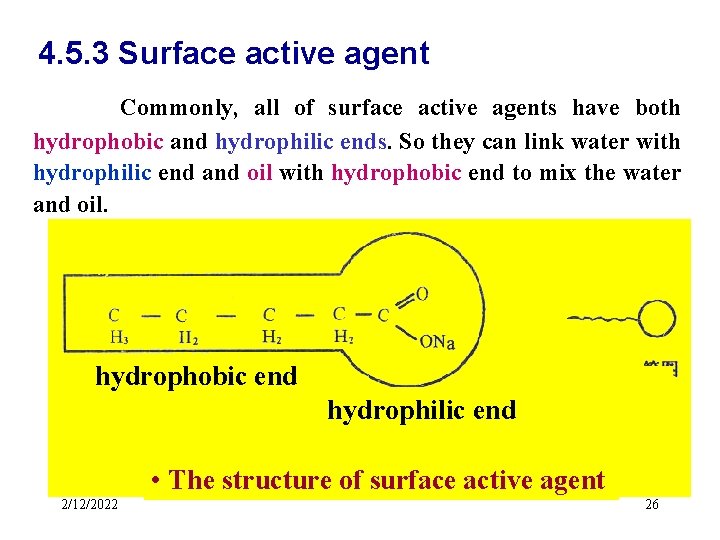
4. 5. 3 Surface active agent Commonly, all of surface active agents have both hydrophobic and hydrophilic ends. So they can link water with hydrophilic end and oil with hydrophobic end to mix the water and oil. hydrophobic end hydrophilic end • The structure of surface active agent 2/12/2022 26

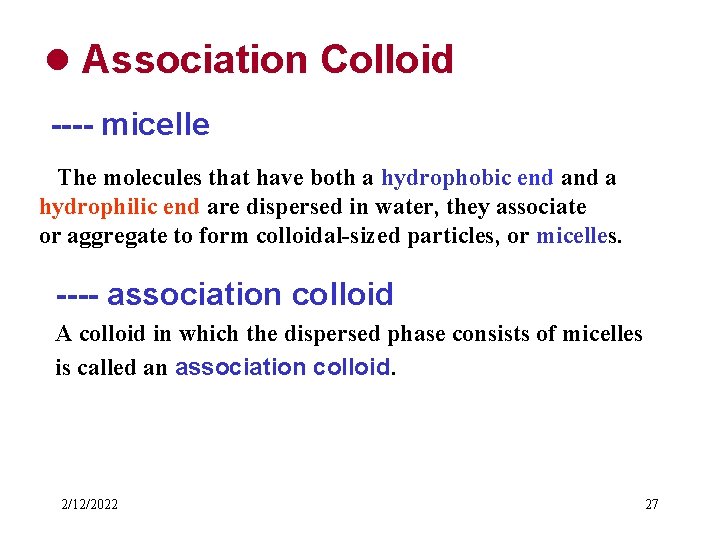
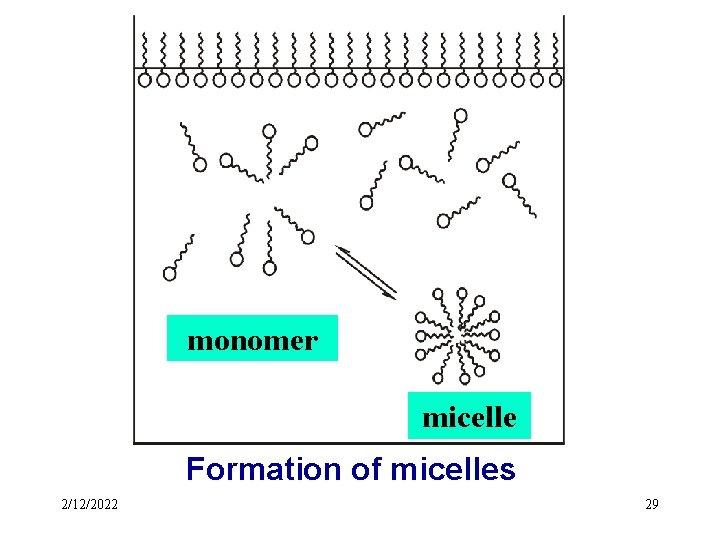
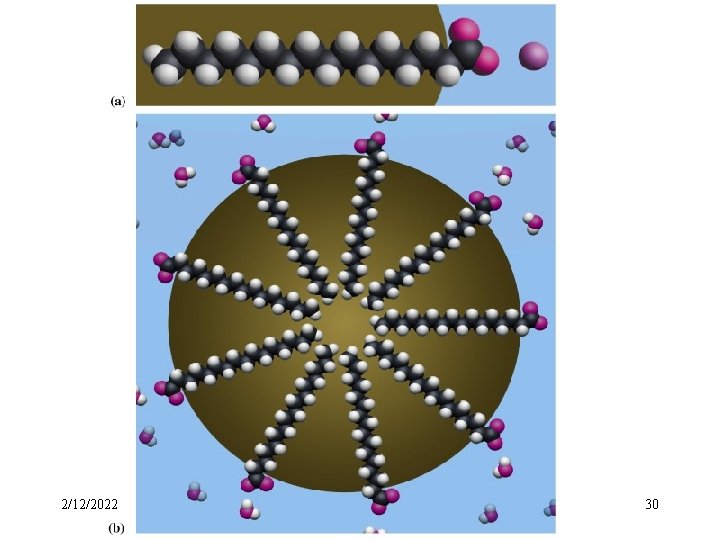
l Association Colloid ---- micelle The molecules that have both a hydrophobic end a hydrophilic end are dispersed in water, they associate or aggregate to form colloidal-sized particles, or micelles. ---- association colloid A colloid in which the dispersed phase consists of micelles is called an association colloid. 2/12/2022 27


monomer micelle Formation of micelles 2/12/2022 29

2/12/2022 30

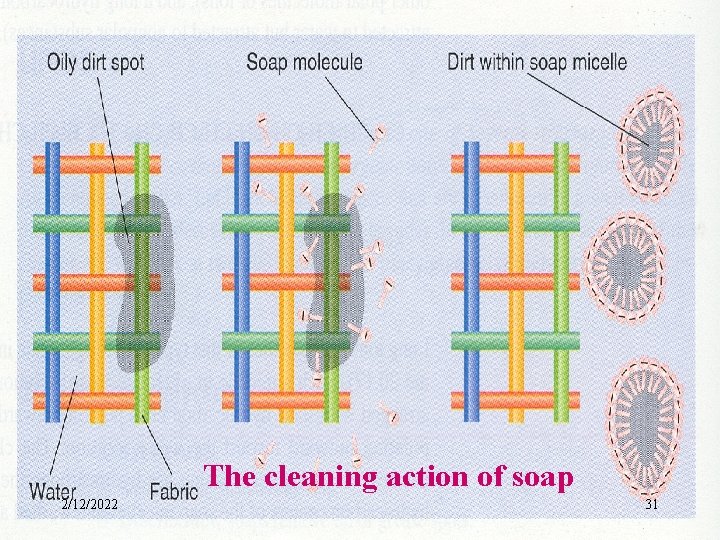
The cleaning action of soap 2/12/2022 31


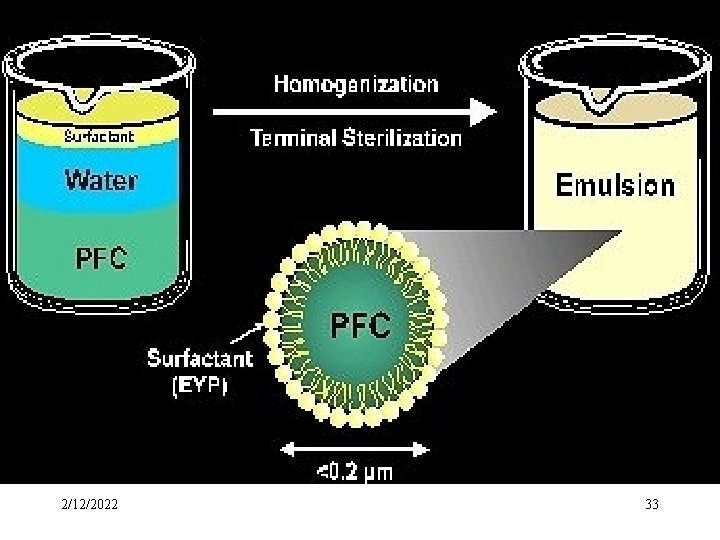
2/12/2022 33

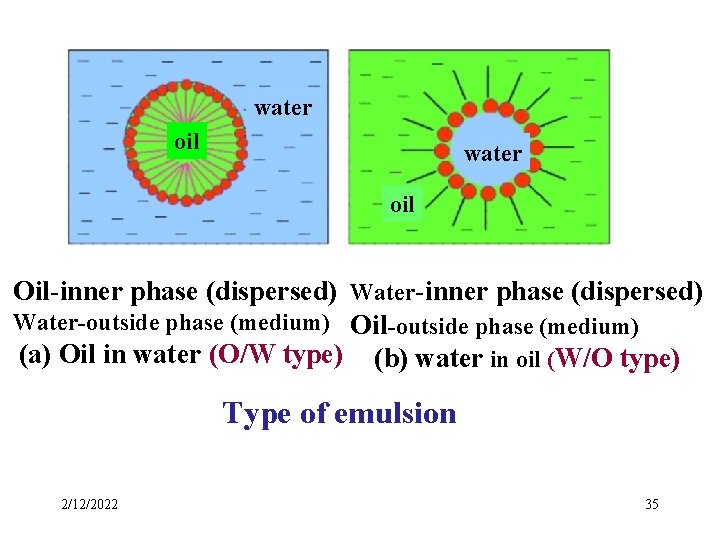
Types of emulsion Emulsions of oil in water (O/W) In which the dispersed phase is the oil while water is the medium. Emulsions of water in oil (W/O) In which the dispersed phase is the water while oil is the medium. 2/12/2022 34

water oil Oil-inner phase (dispersed) Water-outside phase (medium) Oil-outside phase (medium) (a) Oil in water (O/W type) (b) water in oil (W/O type) Type of emulsion 2/12/2022 35






� � (P 77) 4. 13、4. 14、4. 16、4. 18、4. 19 2/12/2022 41