Solution Concentration Which Formula Should I Use Solution












![Practice #3 Binding Buffer is 4 M Ammonium Sulfate [(NH 4)2 SO 4]. How Practice #3 Binding Buffer is 4 M Ammonium Sulfate [(NH 4)2 SO 4]. How](https://slidetodoc.com/presentation_image_h/ed754804d2e424f270a40fcb48f7fd6a/image-16.jpg)








- Slides: 24

Solution Concentration “Which Formula Should I Use? ”

Solution Concentrations Used in Lab • Explicit: g/L or mg/ml • Percent: % • Molar: M, m. M or µM

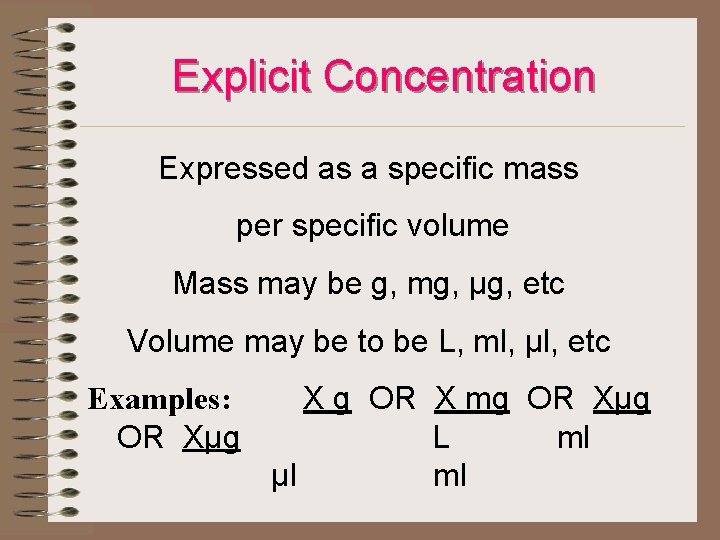
Explicit Concentration Expressed as a specific mass per specific volume Mass may be g, mg, µg, etc Volume may be to be L, ml, µl, etc Examples: X g OR X mg OR Xµg Examples: OR Xµg L ml µl ml

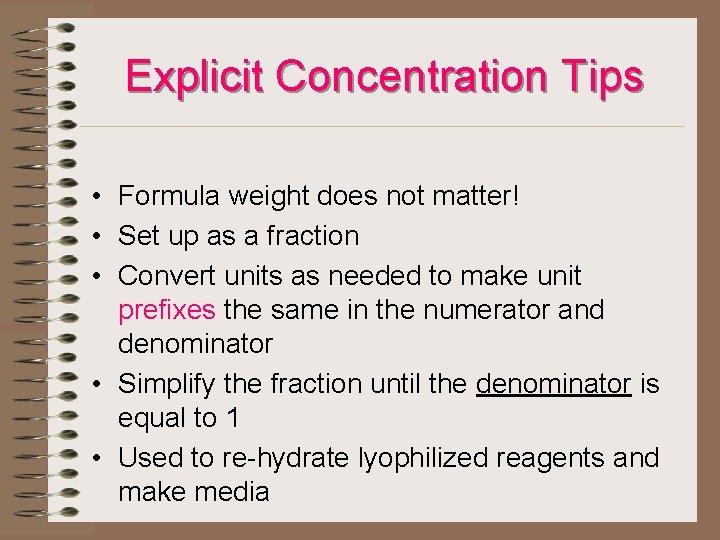
Explicit Concentration Tips • Formula weight does not matter! • Set up as a fraction • Convert units as needed to make unit prefixes the same in the numerator and denominator • Simplify the fraction until the denominator is equal to 1 • Used to re-hydrate lyophilized reagents and make media

Percent Solution X% (w/v) solution = X grams solute 100 ml solution OR X% (v/v) solution = X ml solute 100 ml

Percent Solution Tips • • • Formula weight does not matter! Solid solute indicates w/v Liquid solute indicates v/v Gel concentrations expressed this way Look for ”%”

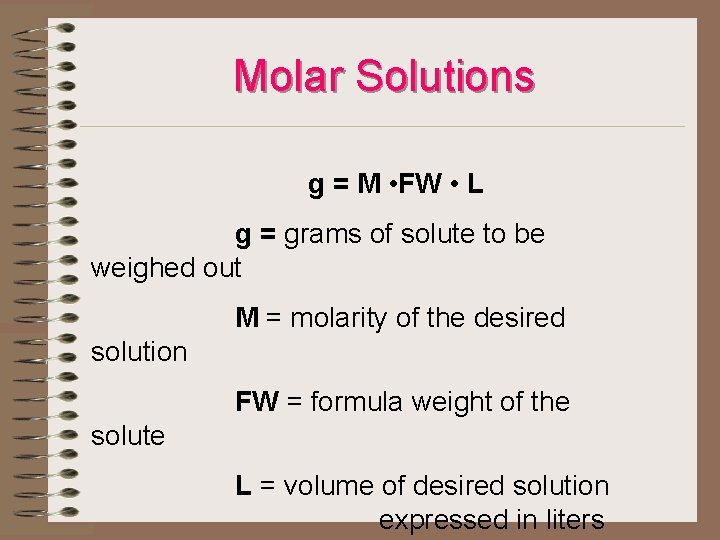
Molar Solutions g = M • FW • L g = grams of solute to be weighed out M = molarity of the desired solution FW = formula weight of the solute L = volume of desired solution expressed in liters

Molar Solutions Tips • • • Formula weight does matter Solute is dry Volume must be expressed in liters Usually solving for “g” Add any water(s) of hydration to the FW of the solute • Look for “M” or “m. M” or “µM”

Diluting Solutions Sometimes you need to dilute a stock solution in order to make a working solution…

Diluting Concentrated Solutions Cs. Vs = Cw. Vw Cs = concentration of stock solution Vs = volume of stock solution needed Cw = desired concentration of working solution Vw = desired volume of working solution

Diluting Solutions Tips • • Stock buffers diluted this way Solute is liquid Volume usually expressed in ml Usually solving for “Vs” • Look for “X”

Practice! Now that you know how to use the formulas, you can practice knowing which formula to use. Continue for practice problems in making solutions in the lab. Read each problem, select which formula you need to apply, and see if you can solve the problem.

Directions Each practice problem will give you four formula choices. Choose the formula that applies to the problem. Then solve the problem yourself. On each “CORRECT!!” slide, click to see the problem set-up, then click again for the solution so you can check your answer. Then click the blue button to go to the

Practice #1 You need to make 1 L of LB/Amp media. The concentration of Amp in the media should be 0. 1 mg/ml. How much Amp powder do you choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml

Practice #2 We purchase PBS as a 20 X stock solution. How would you make 150 ml of 1 X PBS? choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml
![Practice 3 Binding Buffer is 4 M Ammonium Sulfate NH 42 SO 4 How Practice #3 Binding Buffer is 4 M Ammonium Sulfate [(NH 4)2 SO 4]. How](https://slidetodoc.com/presentation_image_h/ed754804d2e424f270a40fcb48f7fd6a/image-16.jpg)
Practice #3 Binding Buffer is 4 M Ammonium Sulfate [(NH 4)2 SO 4]. How would you make 300 ml of this solution? choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml

Practice #4 A 30 mg vial of Ampicillin is rehydrated with 3 ml of sterile distilled water. What is the concentration of ampicillin in this solution? choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml

Practice #5 Methylene Blue is used to stain DNA. How would you make 250 ml of a 0. 5% solution of Methylene Blue using powdered Methylene Blue? choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml

Practice #6 Your protocol calls for a 30 ml, 0. 8% DNA gel. How much agarose powder do you need? choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml

Practice #7 You need 10 m. M EDTA solution to dilute your DNA. How would you make 50 ml of 10 m. M EDTA? choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml

Practice #8 We have 50 X TAE. You need 1 X TAE to run your gel. How would you make 400 ml of 1 X TAE? choose formula: g = M • FW • L Cs. Vs = Cw. Vw X grams solute 100 ml solution mg/ml

Practice #9 How would you prepare 250 ml of a 40 m. M solution of Magnesium Chloride [Mg. Cl 2 • 6 H 2 O]? choose formula: g = M • FW • L Cs. Vs= Cw. Vw X grams solute 100 ml solution mg/ml

Practice #10 We have 2 M glucose solution. How would you make 100 ml of 2 m. M glucose solution? choose formula: g = M • FW • L Cs. Vs= Cw. Vw X grams solute 100 ml solution mg/ml

CONGRATULATIONS! By carefully reading the problems and picking out the unknown, you were able apply the correct formula and do the math!