Solution Characteristics Types Colligative properties Dr N K






- Slides: 14

Solution: Characteristics, Types, Colligative properties Dr. N. K. Shukla Associate Professor Mahatma Gandhi P. G. College Gorakhpur

Solution • A solution is a homogeneous mixture of two or more substances. A solution may exist in any phase. • A solution consists of a solute and a solvent. • The solute is the substance that is dissolved in the solvent. • The amount of solute that can be dissolved in solvent is called its solubility. • For example, in a saline solution, salt is the solute dissolved in water as the solvent. • For solutions with components in the same phase, the substances present in lower concentration are solutes, while the substance present in highest abundance is the solvent. • A dilute solution has a low concentration of the solute compared to the solvent. The opposite of a dilute solution is a concentrated solution, which has high levels of solute in the mixture.

Ideal Solution An ideal solution or ideal mixture is a solution in which the enthalpy of solution (ΔHsolution=0) is zero; with the closer to zero the enthalpy of solution, the more "ideal" the behavior of the solution becomes. The vapor pressure of the solution obeys Raoult's law, and the activity coefficient of each component (which measures deviation from ideality) is equal to one.

Characteristics of a Solution A chemical solution exhibits several properties: • A solution consists of a homogeneous mixture. • A solution is composed of one phase (e. g. , solid, liquid, gas). • Particles in a solution are not visible to the naked eye. • A solution does not scatter a light beam. • Components of a solution cannot be separated using simple mechanical filtration.

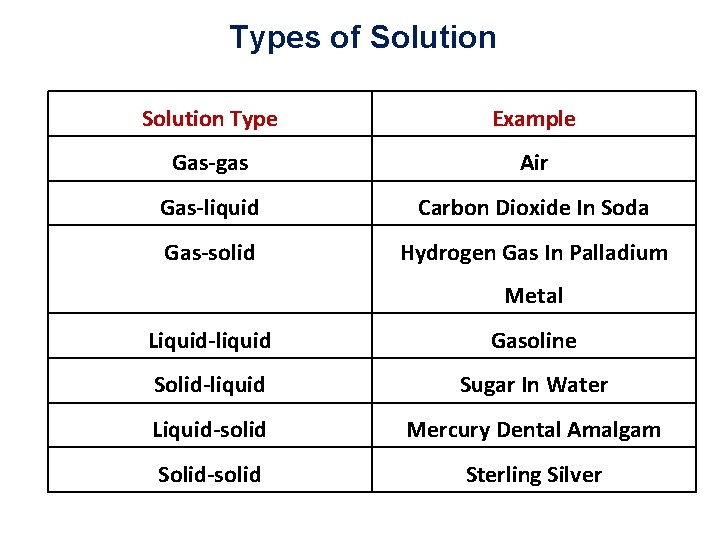
Types of Solution Type Example Gas-gas Air Gas-liquid Carbon Dioxide In Soda Gas-solid Hydrogen Gas In Palladium Metal Liquid-liquid Gasoline Solid-liquid Sugar In Water Liquid-solid Mercury Dental Amalgam Solid-solid Sterling Silver

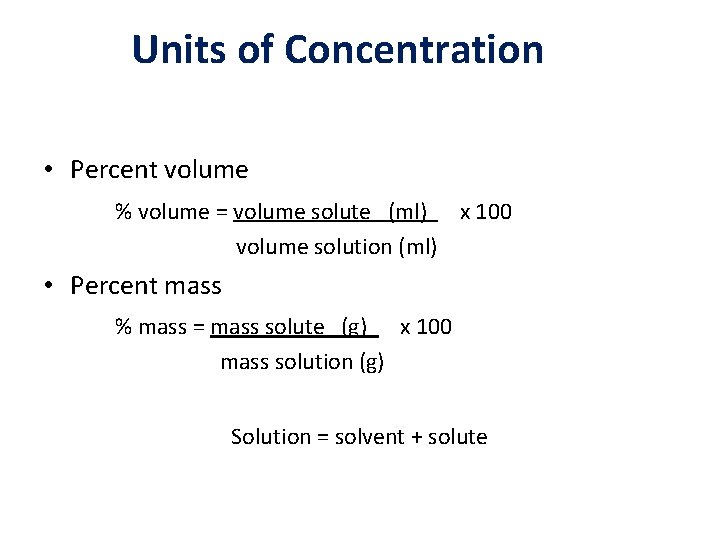
Units of Concentration • Percent volume % volume = volume solute (ml) x 100 volume solution (ml) • Percent mass % mass = mass solute (g) x 100 mass solution (g) Solution = solvent + solute

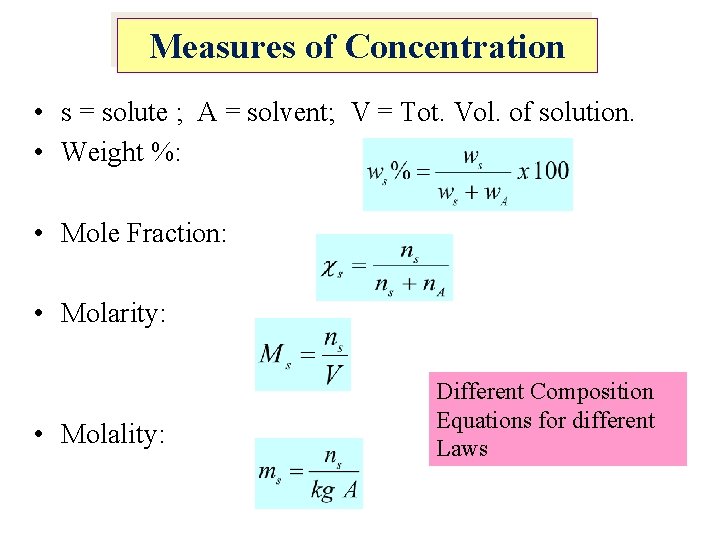
Measures of Concentration • s = solute ; A = solvent; V = Tot. Vol. of solution. • Weight %: • Mole Fraction: • Molarity: • Molality: Different Composition Equations for different Laws

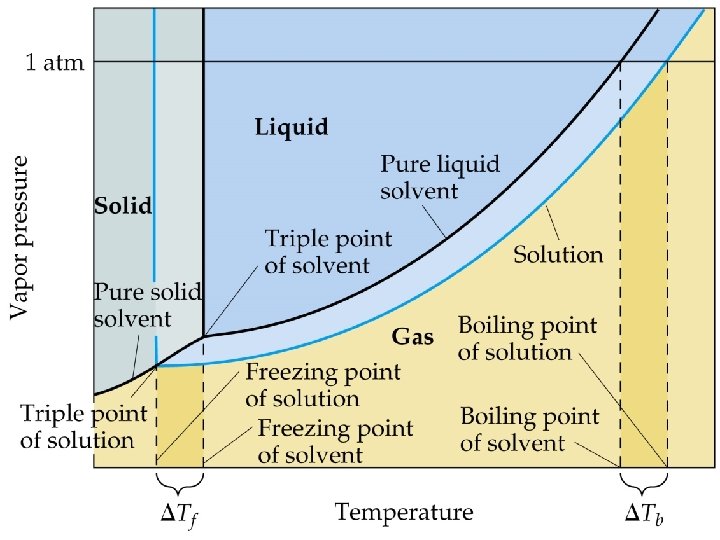
Colligative Properties Colligative properties of solutions are properties that depend upon the concentration of solute molecules or ions, but not upon the identity of the solute. Colligative properties include: Ø vapor pressure lowering, Ø boiling point elevation, Ø freezing point depression, Ø and osmotic pressure.

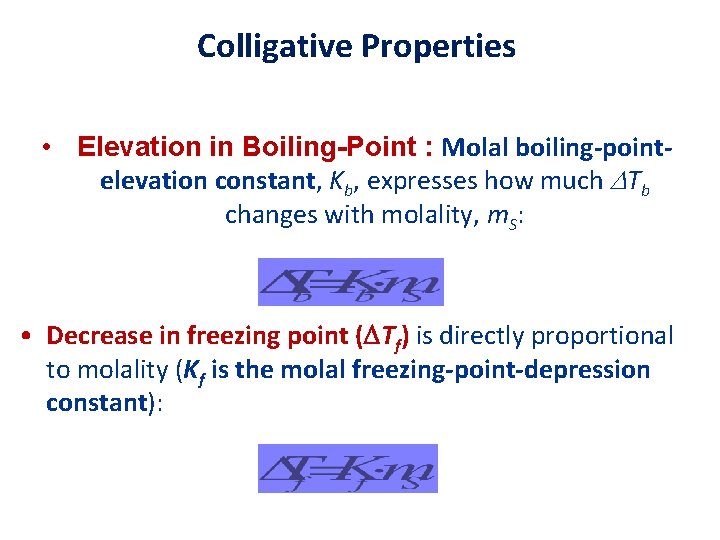
Colligative Properties • Elevation in Boiling-Point : Molal boiling-pointelevation constant, Kb, expresses how much Tb changes with molality, m. S: • Decrease in freezing point ( Tf) is directly proportional to molality (Kf is the molal freezing-point-depression constant):


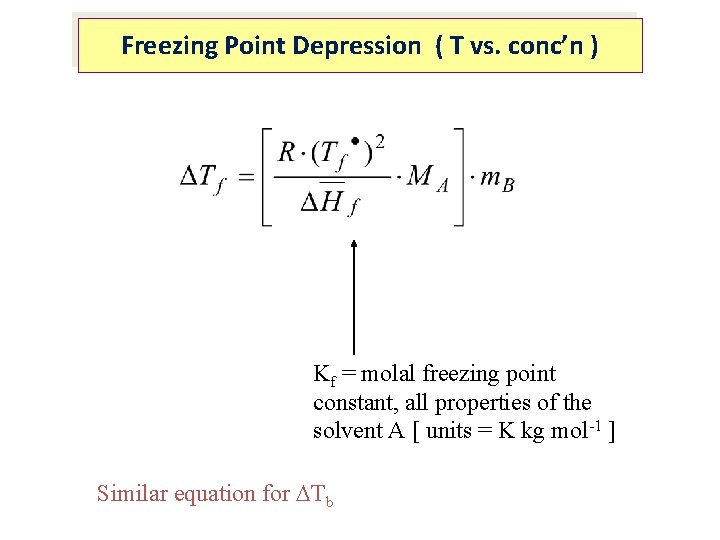
Freezing Point Depression ( T vs. conc’n ) Kf = molal freezing point constant, all properties of the solvent A [ units = K kg mol-1 ] Similar equation for Tb

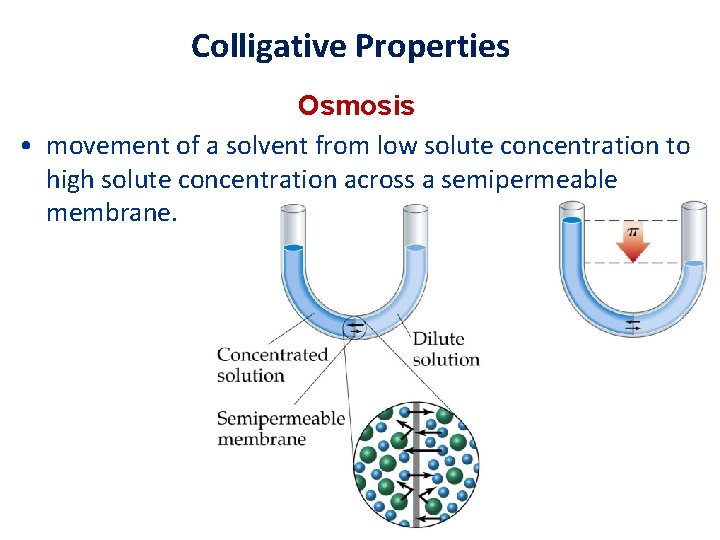
Colligative Properties Osmosis • movement of a solvent from low solute concentration to high solute concentration across a semipermeable membrane.

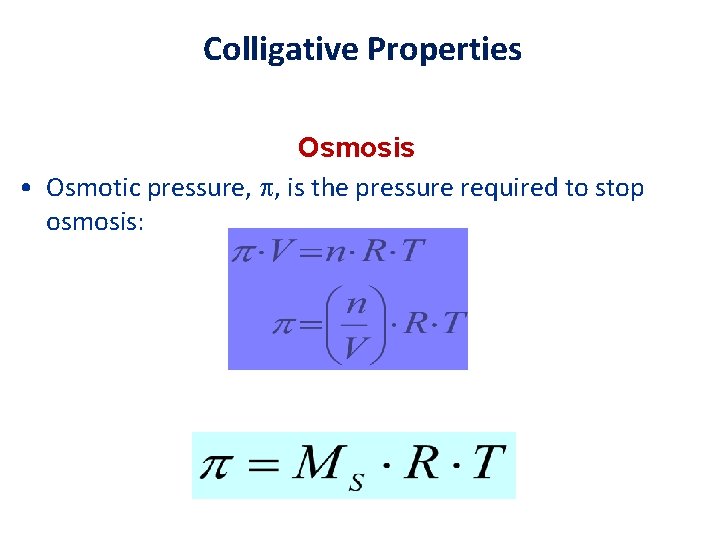
Colligative Properties Osmosis • Osmotic pressure, , is the pressure required to stop osmosis:

References • Physical Chemistry- P. W. Atkins • Physical Chemistry- B. S. Bahl, A. Bahl & G. D. Tuli • Essentials of Physical Chemistry- Pui, Sharma & Pathania