Solute transport in sediments Physical properties Transport Diffusion












- Slides: 35

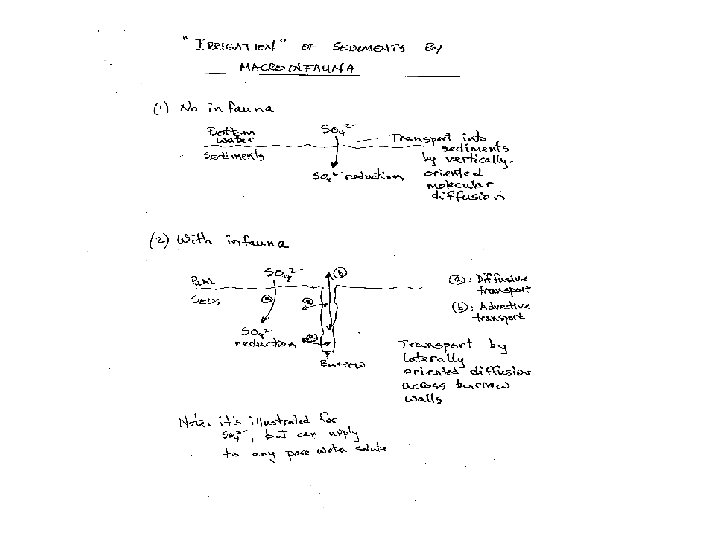
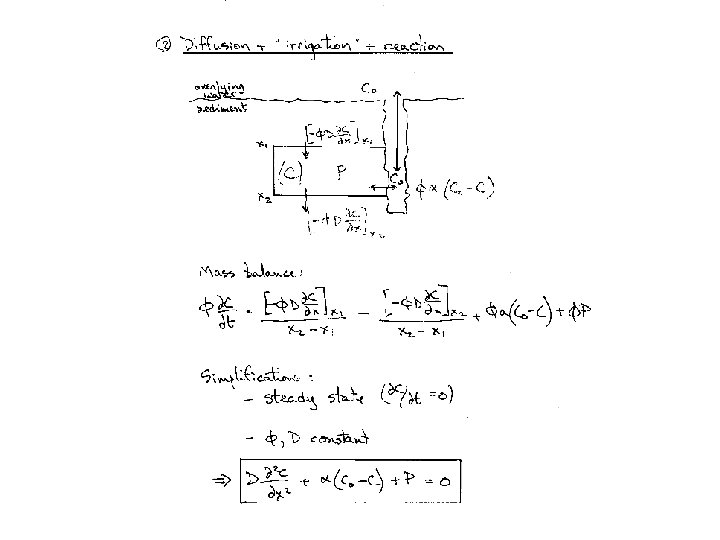
Solute transport in sediments Physical properties Transport: Diffusion “Irrigation” Advection

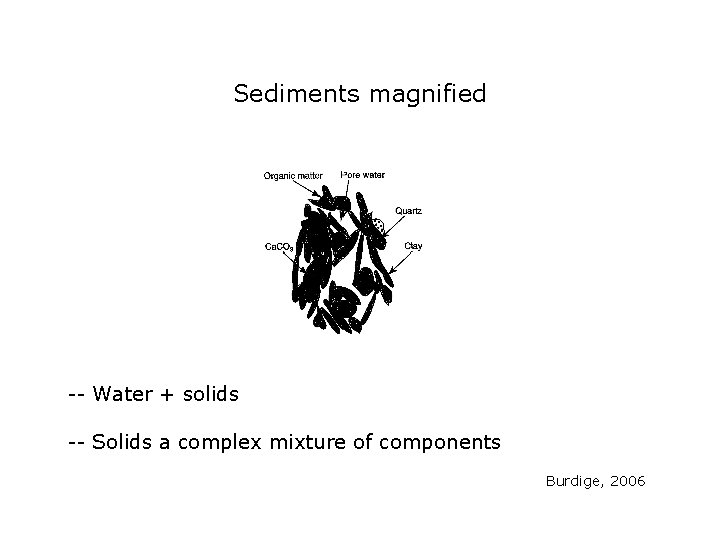
Sediments magnified -- Water + solids -- Solids a complex mixture of components Burdige, 2006

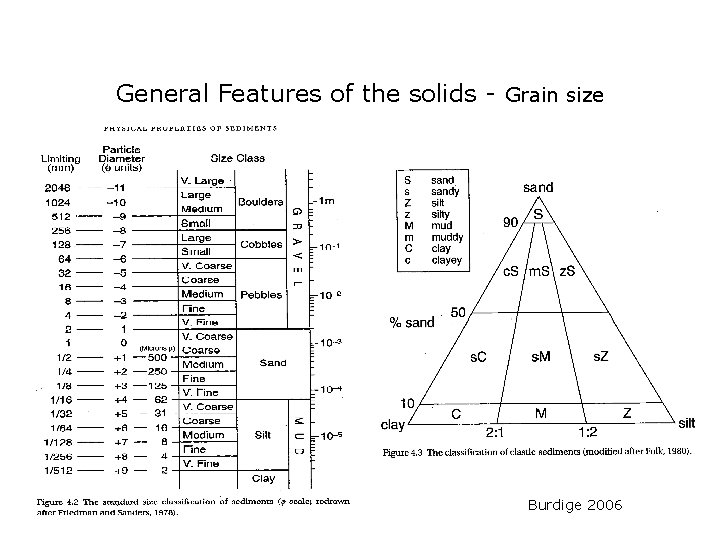
General Features of the solids - Grain size Burdige 2006

Porosity Definition: the volume of connected pore space, per volume of bulk sediment Measurement: measure sediment (1) wet, and (2) after drying in (typically) a 65° oven Then: Typical densities of dry sediments: 2. 6 -2. 7 g/cm 3

Porosity in near surface sediments An example From a typical fine-grained (“clayey silt”) coastal sediment

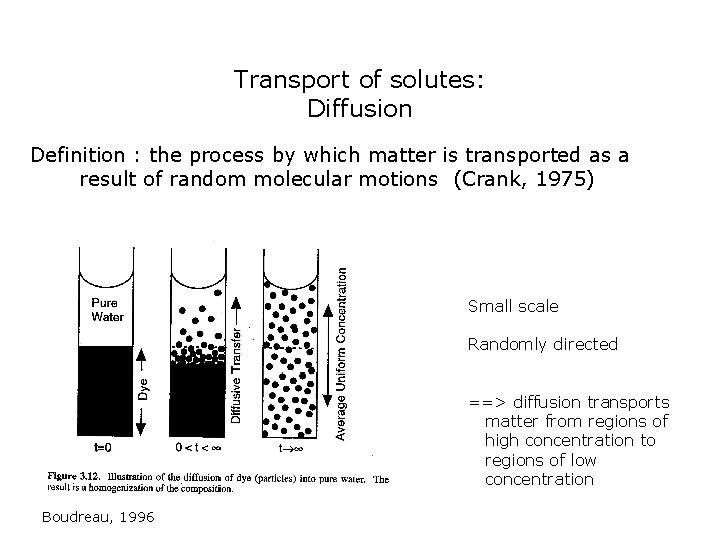
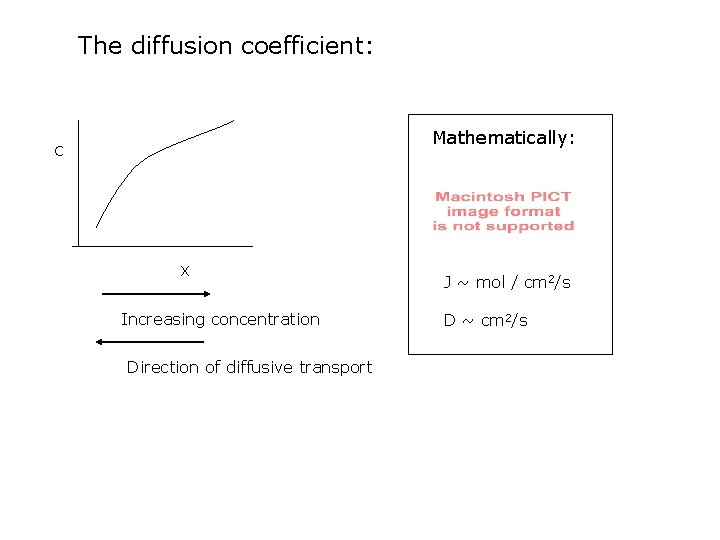
Transport of solutes: Diffusion Definition : the process by which matter is transported as a result of random molecular motions (Crank, 1975) Small scale Randomly directed ==> diffusion transports matter from regions of high concentration to regions of low concentration Boudreau, 1996

The diffusion coefficient: Mathematically: C X Increasing concentration Direction of diffusive transport J ~ mol / cm 2/s D ~ cm 2/s

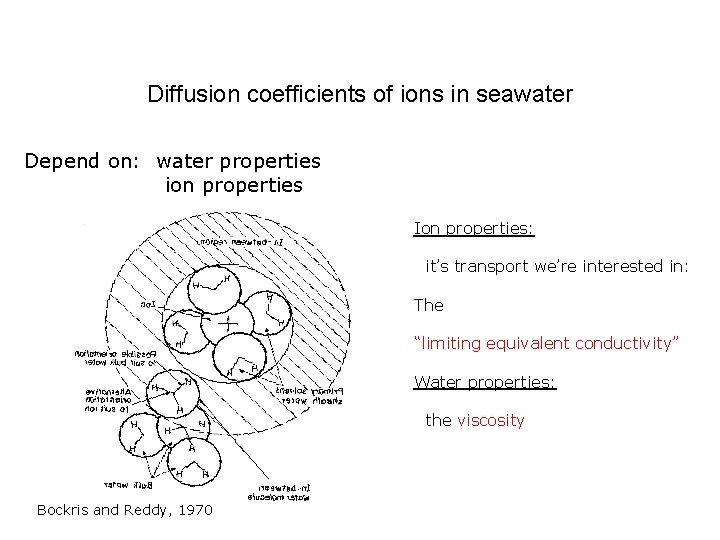
Diffusion coefficients of ions in seawater Depend on: water properties ion properties Ion properties: it’s transport we’re interested in: The “limiting equivalent conductivity” Water properties: the viscosity Bockris and Reddy, 1970

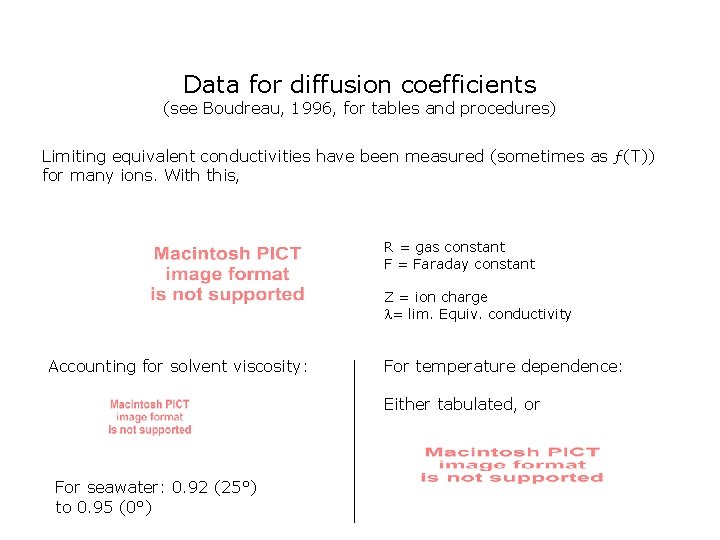
Data for diffusion coefficients (see Boudreau, 1996, for tables and procedures) Limiting equivalent conductivities have been measured (sometimes as ƒ(T)) for many ions. With this, R = gas constant F = Faraday constant Z = ion charge = lim. Equiv. conductivity Accounting for solvent viscosity: For temperature dependence: Either tabulated, or For seawater: 0. 92 (25°) to 0. 95 (0°)

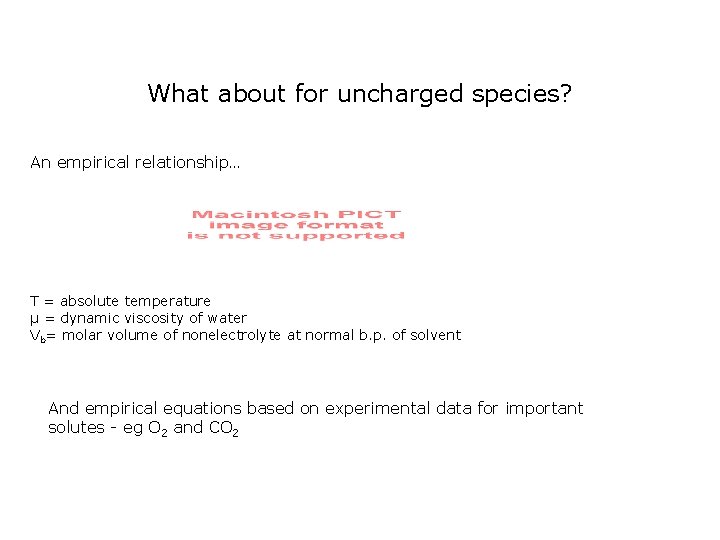
What about for uncharged species? An empirical relationship… T = absolute temperature µ = dynamic viscosity of water Vb= molar volume of nonelectrolyte at normal b. p. of solvent And empirical equations based on experimental data for important solutes - eg O 2 and CO 2

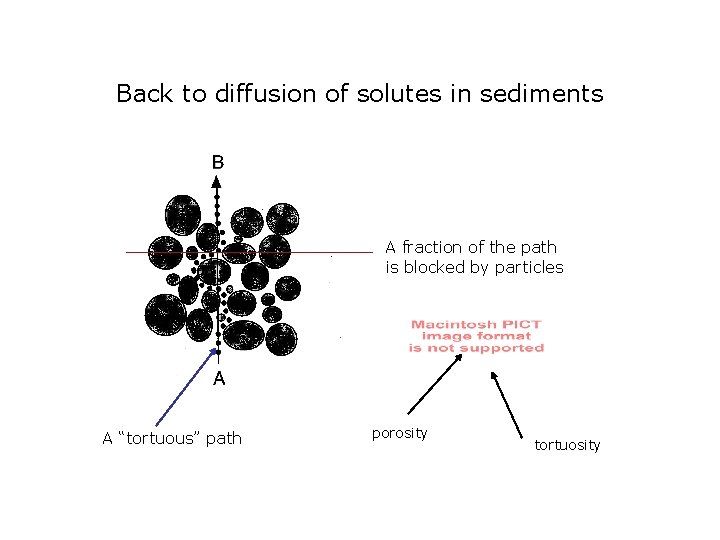
Back to diffusion of solutes in sediments A fraction of the path is blocked by particles A “tortuous” path porosity tortuosity

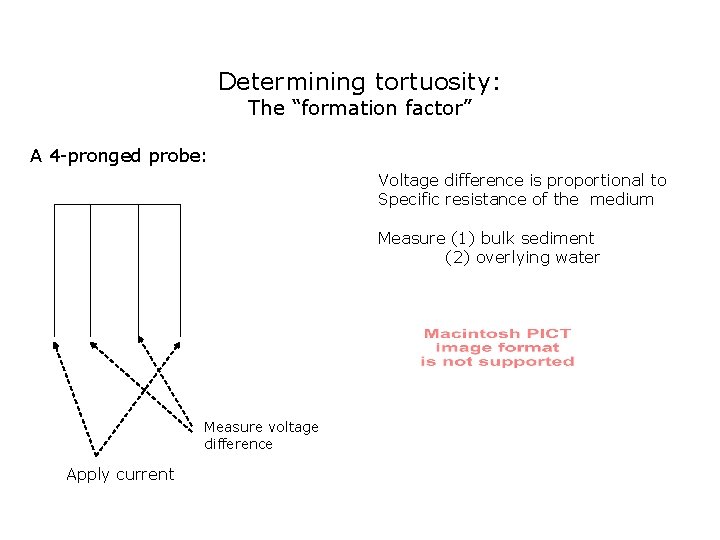
Determining tortuosity: The “formation factor” A 4 -pronged probe: Voltage difference is proportional to Specific resistance of the medium Measure (1) bulk sediment (2) overlying water Measure voltage difference Apply current

Example F is closely related to porosity for a given Sediment: C~1; n~2 -4

Finally… The diffusive flux in a porous medium:

One last remark A general, qualitative relationship… Can be derived from consideration of diffusion as a “random walk” The “average” (root mean square) distance of transport of a substance by diffusion in the time interval, t:

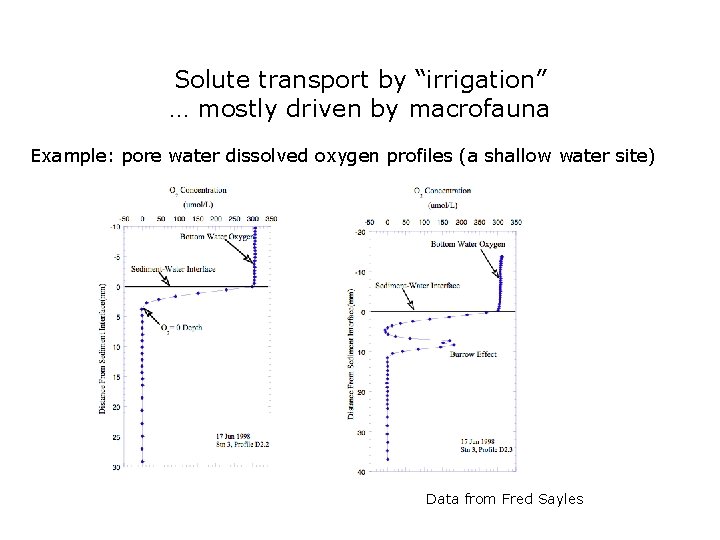
Solute transport by “irrigation” … mostly driven by macrofauna Example: pore water dissolved oxygen profiles (a shallow water site) Data from Fred Sayles

Comparing fluxes: Diffusive fluxes from pore water profies vs Fluxes from in situ benthic flux chambers Location: Massachusetts Bay, water depth 35 m

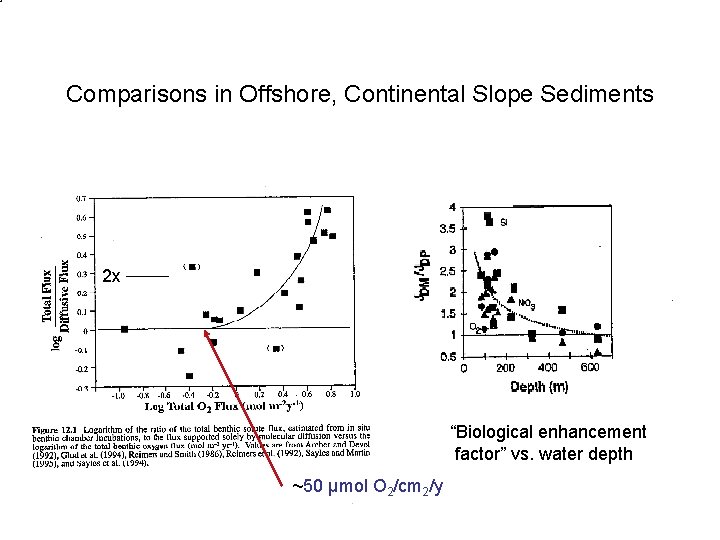
Comparisons in Offshore, Continental Slope Sediments 2 x “Biological enhancement factor” vs. water depth ~50 µmol O 2/cm 2/y

Organic C oxidation rates Continental Margin, NW Atlantic

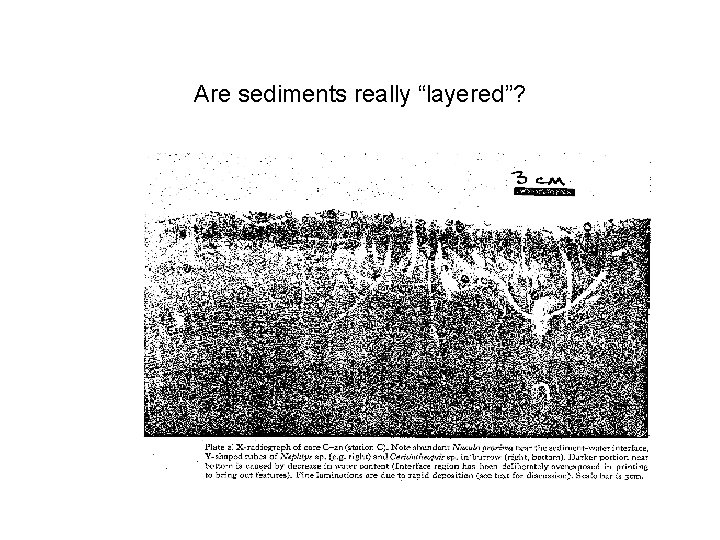
Are sediments really “layered”?




Example: Rn-222 deficits in Buzzards Bay: winter: alpha ~ 0 spring / summer: alpha > 0

Rn results: Continental slope Filled : Rn-222 production rate Open: Rn-222 measured in pore water Dashed line: Rn-222 model: diffusion only

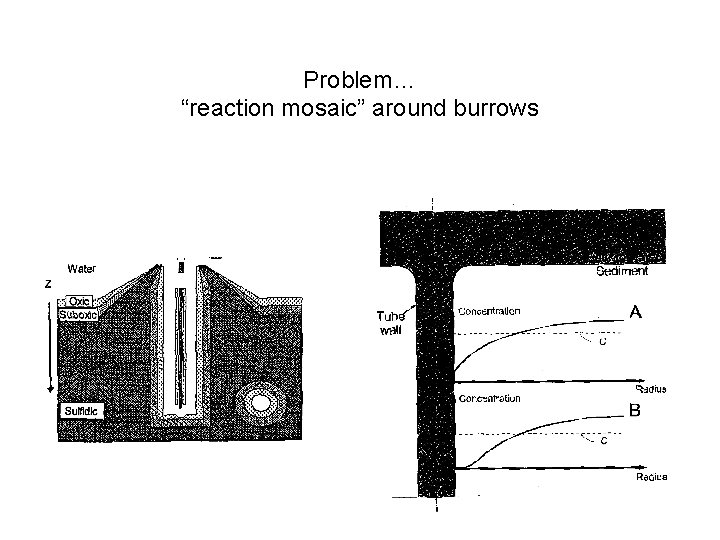
Problem… “reaction mosaic” around burrows

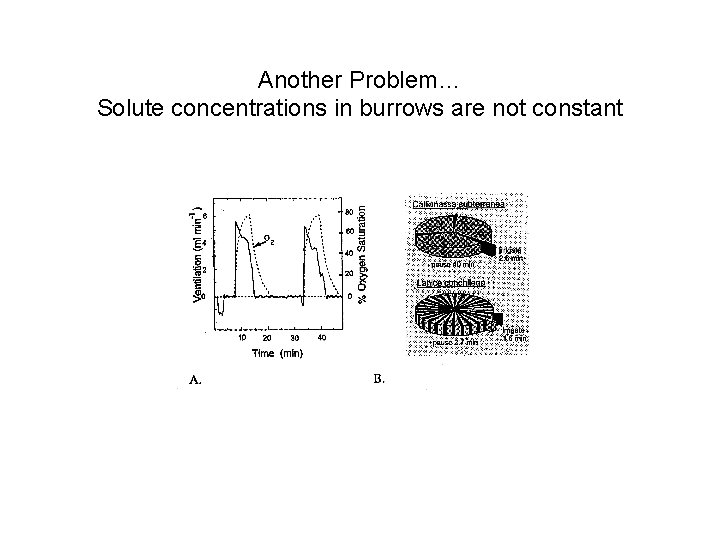
Another Problem… Solute concentrations in burrows are not constant

And: 1. Flushing can affect reaction rates e. g. : removal of products 2. Burrows are active environments on their own

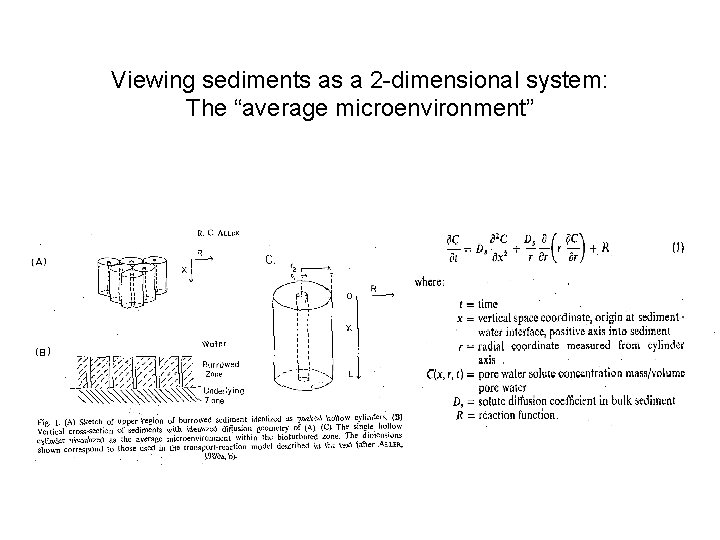
Viewing sediments as a 2 -dimensional system: The “average microenvironment”

…Applying the 2 -D model to data… Parameters: r 1 = 0. 05 cm: est. based on numerically dominant poychaete L = 15 cm: est. from x-radiographs R = based on incubations for SO 4 and NH 4+; for Si. O 2, assume Ceq = 577µM; vary rate constant to fit r 2 = 2. 1 cm: varied to fit NH 4+ profile; used for other 2 solutes

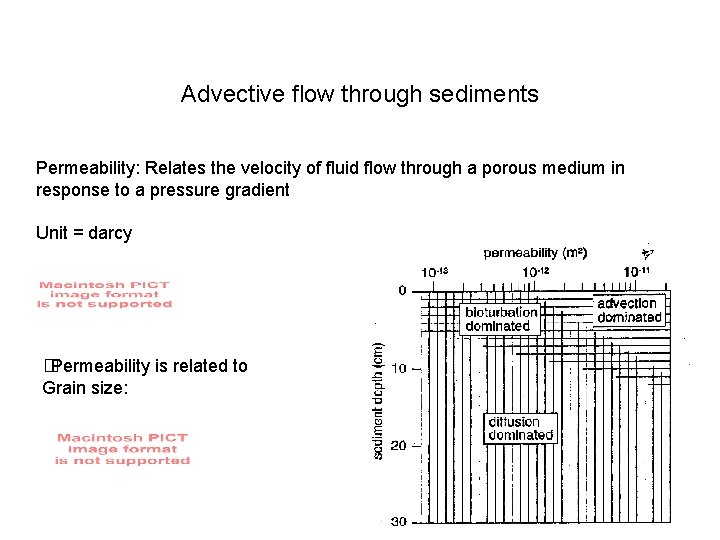
Advective flow through sediments Permeability: Relates the velocity of fluid flow through a porous medium in response to a pressure gradient Unit = darcy � Permeability is related to Grain size:

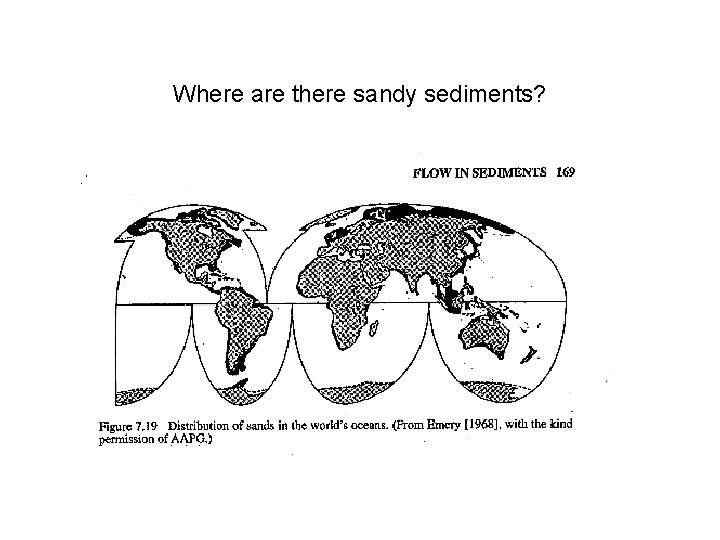
Where are there sandy sediments?

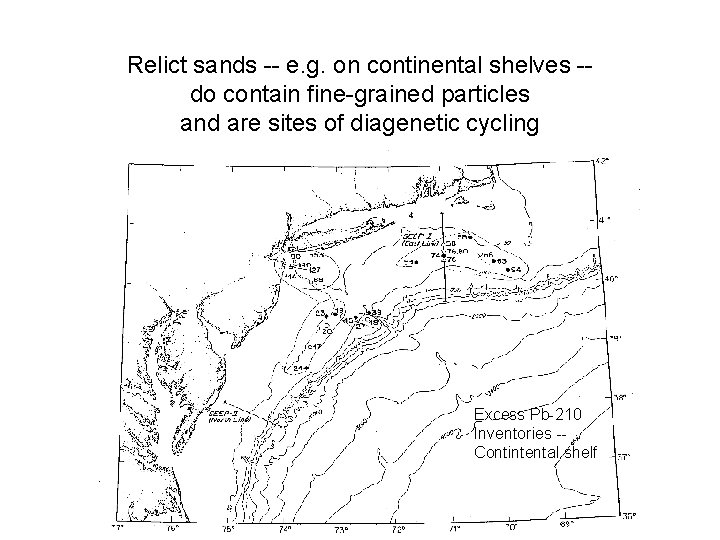
Relict sands -- e. g. on continental shelves -do contain fine-grained particles and are sites of diagenetic cycling Excess Pb-210 Inventories -Contintental shelf

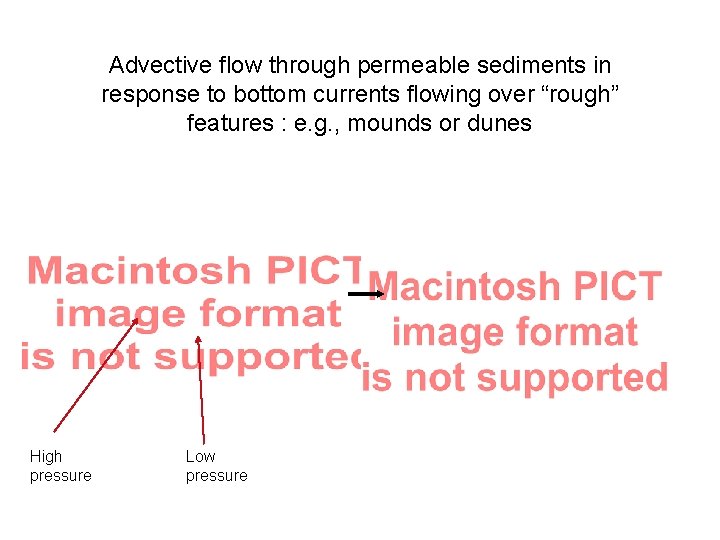
Advective flow through permeable sediments in response to bottom currents flowing over “rough” features : e. g. , mounds or dunes High pressure Low pressure

Flow + filtering of particles from flow … Measurements of dissolved Oxygen around a mound -Results from experiment in a flume