Solubility UNSATURATED SOLUTION more solute dissolves SATURATED SOLUTION
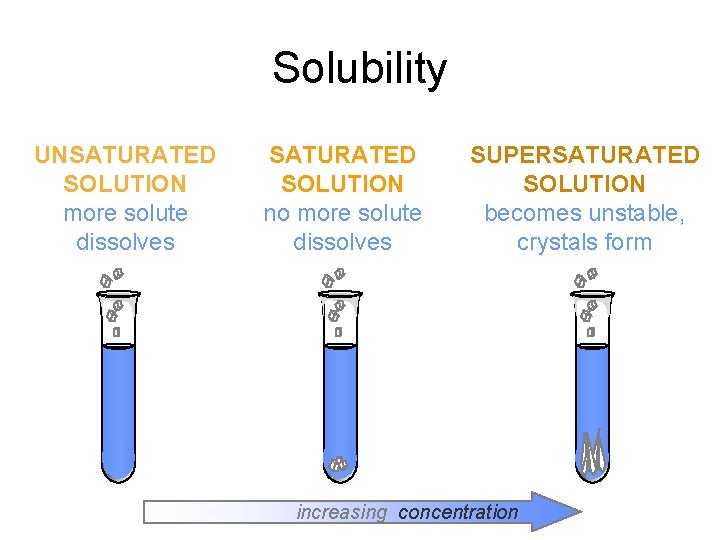
Solubility UNSATURATED SOLUTION more solute dissolves SATURATED SOLUTION no more solute dissolves SUPERSATURATED SOLUTION becomes unstable, crystals form increasing concentration

Solubility vs. Temperature for Solids 140 KI 130 Solubility Table shows the dependence of solubility on temperature Solubility (grams of solute/100 g H 2 O) 120 Na. NO 3 110 gases solids 100 KNO 3 90 80 HCl 70 60 NH 3 KCl 50 40 30 Na. Cl 20 10 KCl. O 3 SO 2 0 Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 517 NH 4 Cl 10 20 30 40 50 60 70 80 90 100

Solubility maximum grams of solute that will dissolve in 100 g of solvent at a given temperature varies with temperature based on a saturated solution
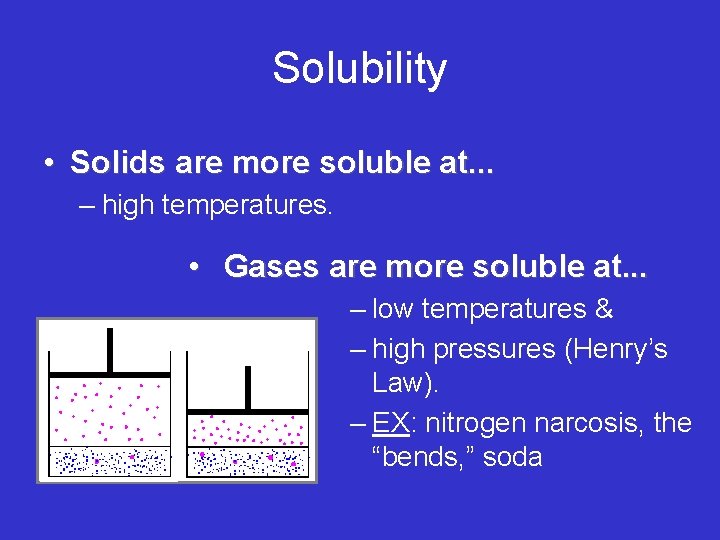
Solubility • Solids are more soluble at. . . – high temperatures. • Gases are more soluble at. . . – low temperatures & – high pressures (Henry’s Law). – EX: nitrogen narcosis, the “bends, ” soda
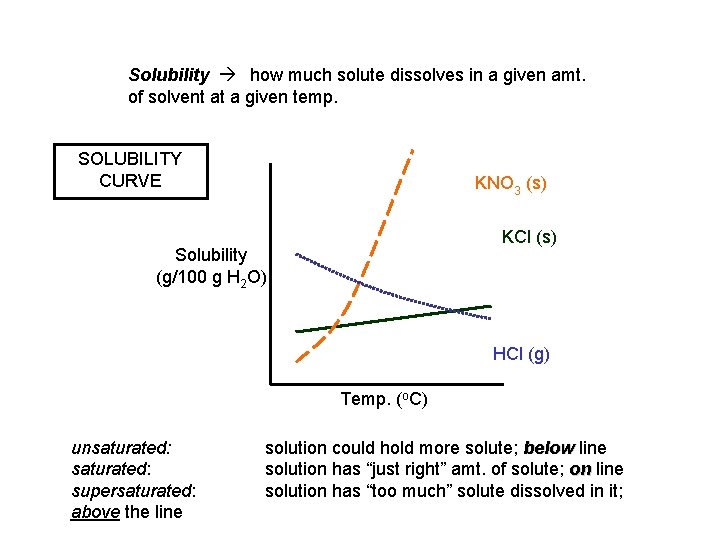
Solubility how much solute dissolves in a given amt. of solvent at a given temp. SOLUBILITY CURVE KNO 3 (s) KCl (s) Solubility (g/100 g H 2 O) HCl (g) Temp. (o. C) unsaturated: supersaturated: above the line solution could hold more solute; below line solution has “just right” amt. of solute; on line solution has “too much” solute dissolved in it;
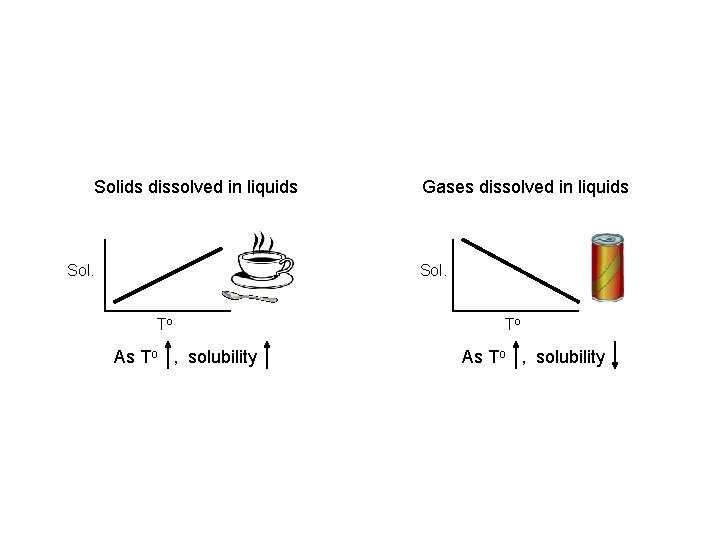
Solids dissolved in liquids Sol. Gases dissolved in liquids Sol. To As To , solubility
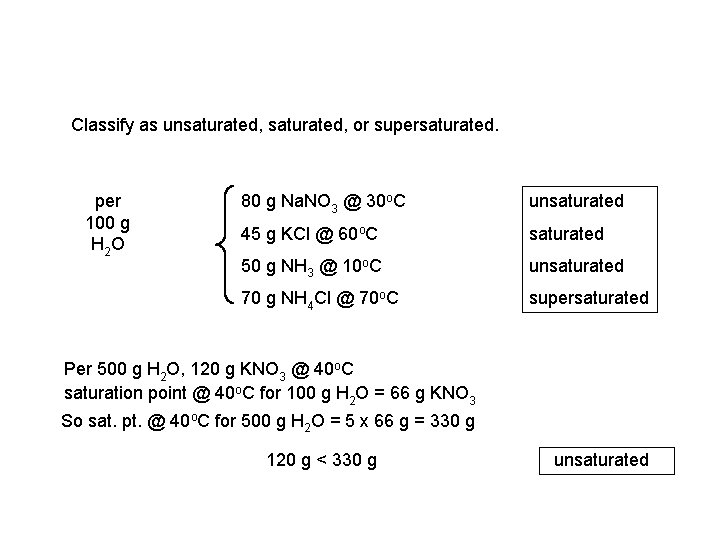
Classify as unsaturated, or supersaturated. per 100 g H 2 O 80 g Na. NO 3 @ 30 o. C unsaturated 45 g KCl @ 60 o. C saturated 50 g NH 3 @ 10 o. C unsaturated 70 g NH 4 Cl @ 70 o. C supersaturated Per 500 g H 2 O, 120 g KNO 3 @ 40 o. C saturation point @ 40 o. C for 100 g H 2 O = 66 g KNO 3 So sat. pt. @ 40 o. C for 500 g H 2 O = 5 x 66 g = 330 g 120 g < 330 g unsaturated
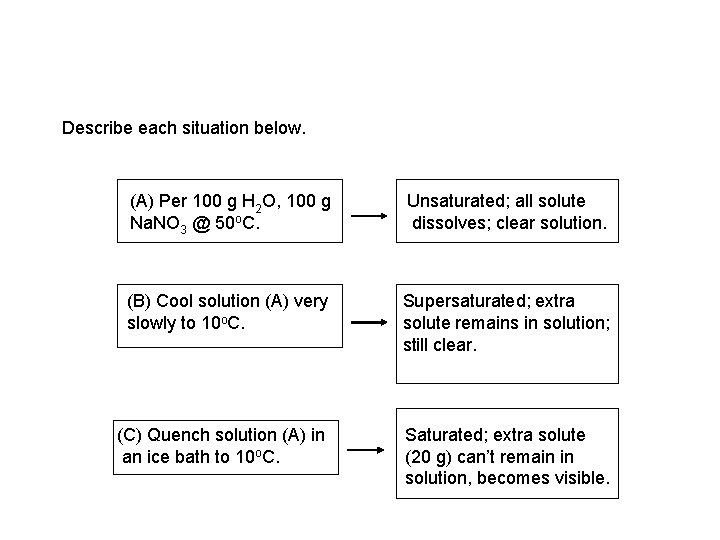
Describe each situation below. (A) Per 100 g H 2 O, 100 g Na. NO 3 @ 50 o. C. Unsaturated; all solute dissolves; clear solution. (B) Cool solution (A) very slowly to 10 o. C. Supersaturated; extra solute remains in solution; still clear. (C) Quench solution (A) in an ice bath to 10 o. C. Saturated; extra solute (20 g) can’t remain in solution, becomes visible.
Hotpack / Coldpack

Solubility of Sodium Acetate Solubility(g/100 g H 2 O) 150 Saturated Supersaturated solution 100 Unsaturated solution 50 Video Clip 0 25 50 75 Temperature (o. C) Charles H. Corwin, Introductory Chemistry 2005, page 378 100 The small crystal A single crystal ofcauses sodiumextensive acetate, crystallization, and eventually Na. C 2 H 3 O 2, is dropped into a supersatureated solution the solute forms a solid mass of Na. C 2 H 3 O 2.

Vitamins • Multi Vitamin – Provides many essential vitamins – “Expensive urine” • Water Soluble – Vitamin C – Must be replenished regularly • Fat Soluble – Can overdose – Vitamin A – Can be ingested periodically, stored in body fat
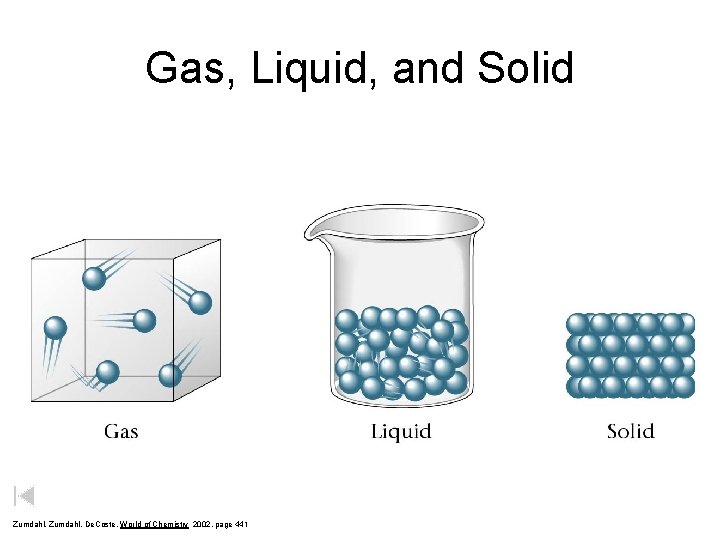
Gas, Liquid, and Solid Zumdahl, De. Coste, World of Chemistry 2002, page 441
- Slides: 12