Solubility Unit III Lesson 1 Solubility is the

Solubility Unit III Lesson 1

Solubility is the maximum amount of solute that will dissolve in a volume of water. Units: g/L mol/L g/100 m. L The unit must have an amount on the top and volume on the bottom! In order to determine the solubility you must completely fill or saturate the solution! To saturate a solution, add weighed portions of the solid to a volume of water and stir until full. A bit of excess solid is present when saturated.
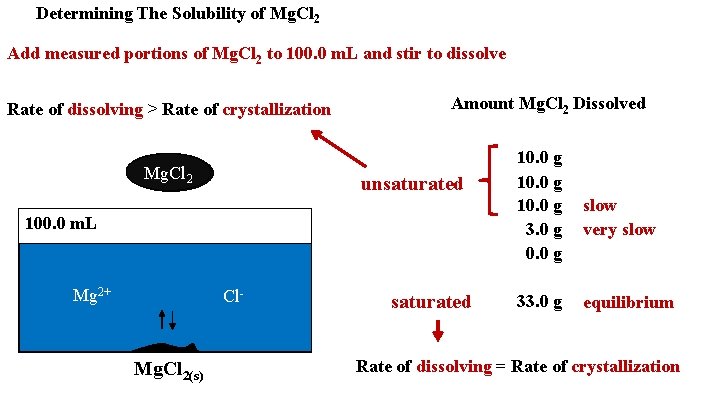
Determining The Solubility of Mg. Cl 2 Add measured portions of Mg. Cl 2 to 100. 0 m. L and stir to dissolve Rate of dissolving > Rate of crystallization Mg. Cl 22 Amount Mg. Cl 2 Dissolved unsaturated 100. 0 m. L Mg 2+ Cl- Mg. Cl 2(s) saturated 10. 0 g 3. 0 g 0. 0 g 33. 0 g slow very slow equilibrium Rate of dissolving = Rate of crystallization
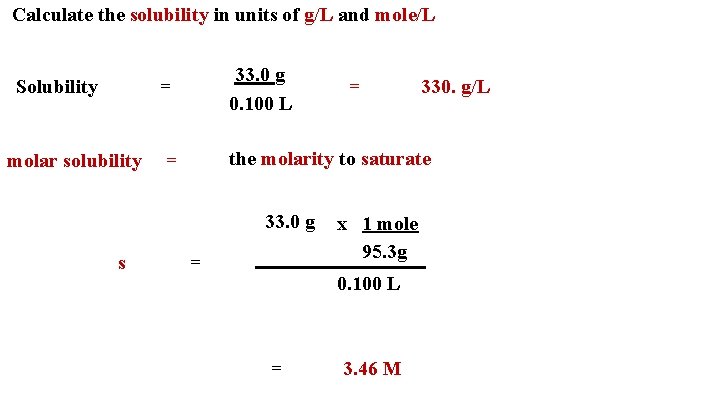
Calculate the solubility in units of g/L and mole/L Solubility 33. 0 g 0. 100 L = 330. g/L the molarity to saturate molar solubility = 33. 0 g s = = x 1 mole 95. 3 g 0. 100 L = 3. 46 M
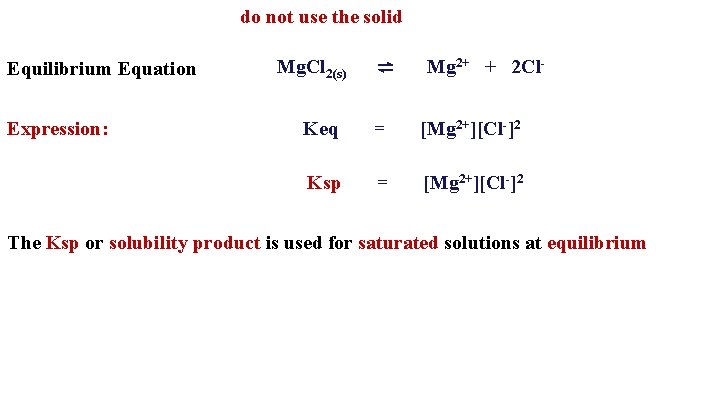
do not use the solid Equilibrium Equation Expression: Mg. Cl 2(s) ⇌ Mg 2+ + 2 Cl- Keq = [Mg 2+][Cl-]2 Ksp = [Mg 2+][Cl-]2 The Ksp or solubility product is used for saturated solutions at equilibrium

This unit is all about the Ksp and the solubility of saturated solutions.

Unsaturated Solutions Not full -more solid dissolves The rate of dissolving > the rate of crystallizing Not at equilibrium

Saturated Solutions Full-more solid does not dissolve The rate of dissolving = the rate of crystallizing At equilibrium

Supersaturated Solutions Over full -more solid causes precipitation The rate of dissolving < the rate of crystallizing Not at equilibrium
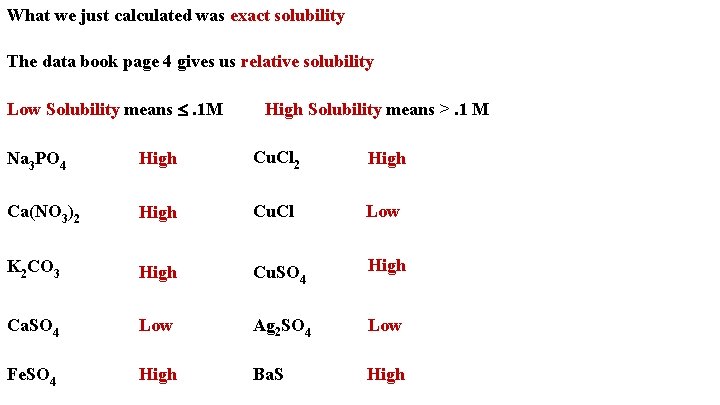
What we just calculated was exact solubility The data book page 4 gives us relative solubility Low Solubility means . 1 M High Solubility means >. 1 M Na 3 PO 4 High Cu. Cl 2 High Ca(NO 3)2 High Cu. Cl Low K 2 CO 3 High Cu. SO 4 High Ca. SO 4 Low Ag 2 SO 4 Low Fe. SO 4 High Ba. S High
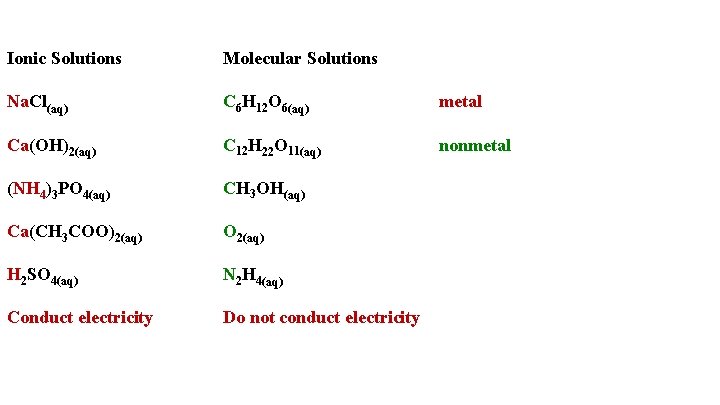
Ionic Solutions Na. Cl(aq) Ca(OH)2(aq) (NH 4)3 PO 4(aq) Ca(CH 3 COO)2(aq) H 2 SO 4(aq) Conduct electricity Molecular Solutions C 6 H 12 O 6(aq) metal C 12 H 22 O 11(aq) nonmetal CH 3 OH(aq) O 2(aq) N 2 H 4(aq) Do not conduct electricity
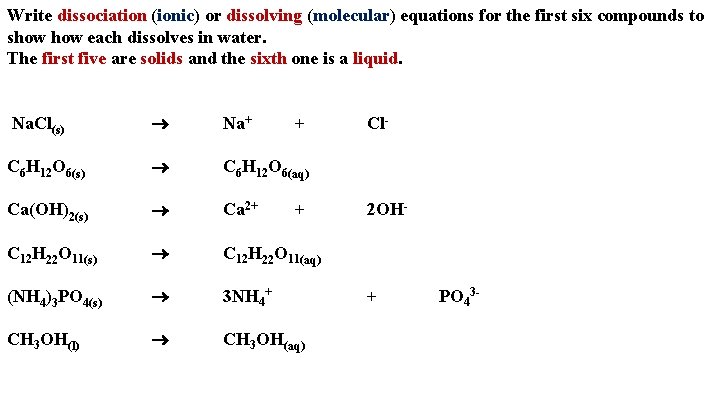
Write dissociation (ionic) or dissolving (molecular) equations for the first six compounds to show each dissolves in water. The first five are solids and the sixth one is a liquid. Na. Cl(s) Na+ C 6 H 12 O 6(s) C 6 H 12 O 6(aq) Ca(OH)2(s) Ca 2+ C 12 H 22 O 11(s) C 12 H 22 O 11(aq) (NH 4)3 PO 4(s) 3 NH 4+ CH 3 OH(l) CH 3 OH(aq) + + Cl- 2 OH- + PO 43 -
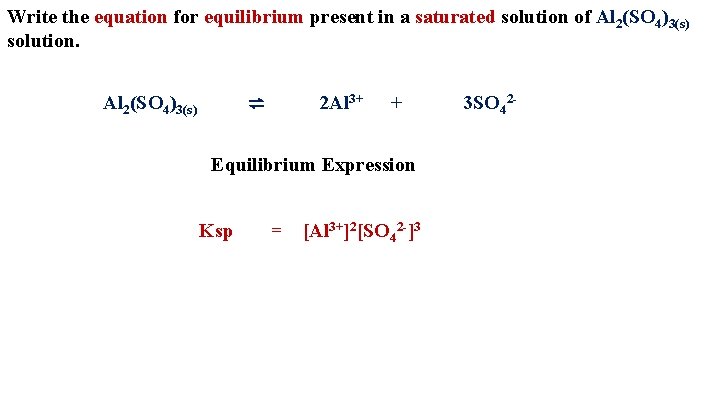
Write the equation for equilibrium present in a saturated solution of Al 2(SO 4)3(s) solution. ⇌ Al 2(SO 4)3(s) 2 Al 3+ + Equilibrium Expression Ksp = [Al 3+]2[SO 42 -]3 3 SO 42 -
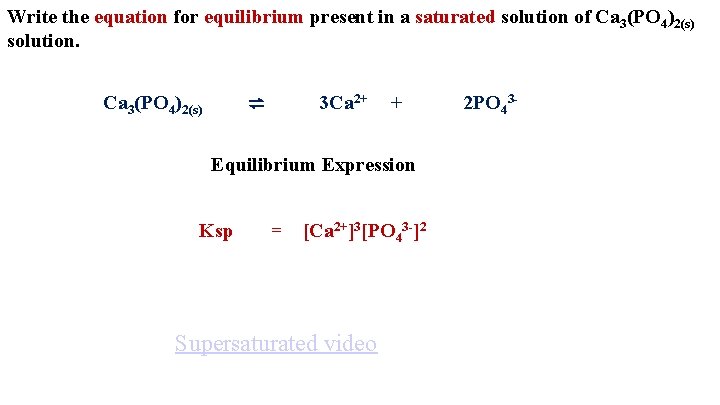
Write the equation for equilibrium present in a saturated solution of Ca 3(PO 4)2(s) solution. ⇌ Ca 3(PO 4)2(s) 3 Ca 2+ + Equilibrium Expression Ksp = [Ca 2+]3[PO 43 -]2 Supersaturated video 2 PO 43 -
- Slides: 14