Solubility The maximum quantity of the substance expressed

Solubility The maximum quantity of the substance, expressed in grams, that will dissolve in a certain solvent at a specific temperature. S-C-9 -1_Solubility Presentation
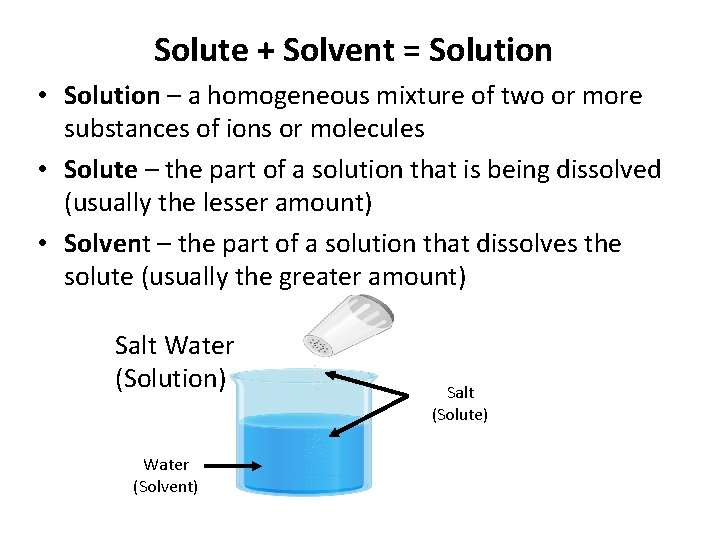
Solute + Solvent = Solution • Solution – a homogeneous mixture of two or more substances of ions or molecules • Solute – the part of a solution that is being dissolved (usually the lesser amount) • Solvent – the part of a solution that dissolves the solute (usually the greater amount) Salt Water (Solution) Water (Solvent) Salt (Solute)
Types of Solutions • Saturated – a solution that contains the maximum quantity of solute that dissolves at that temperature. • Unsaturated – a solution that contains less than the maximum amount of solute that can dissolve at a particular temperature. • Supersaturated – a solution that contains more solute than a saturated solution. – Supersaturated solutions can be attained by heating the solution up to dissolve more solute at that higher temperature and then letting the solution cool. – Once cooled, agitation causes crystals to precipitate (separate from solution) out of the super saturated solution. S-C-9 -1_Solubility Presentation
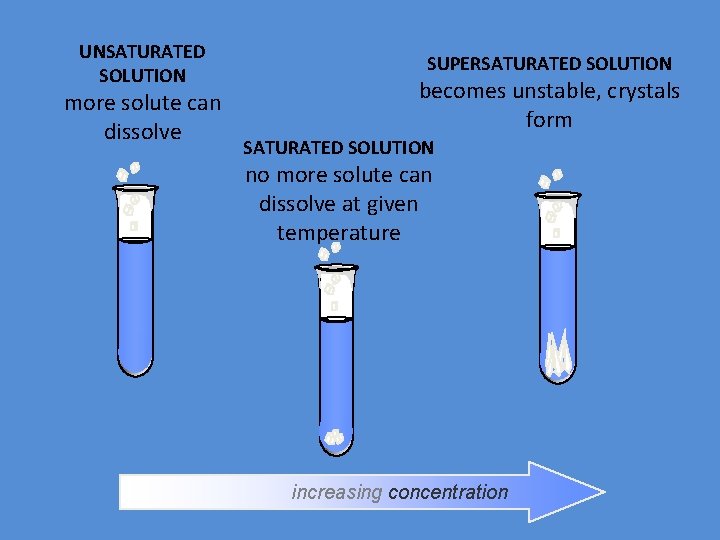
UNSATURATED SOLUTION more solute can dissolve SUPERSATURATED SOLUTION becomes unstable, crystals form SATURATED SOLUTION no more solute can dissolve at given temperature increasing concentration
Solubility and Temperature Increasing the temperature of a solvent speeds up the particle movement. This causes more solvent particles to bump into the solute, resulting in solute particles breaking loose and dissolving faster. Solubility Curve A graph of the solubility of a compound (grams/100 grams water on the Y-axis) at various temperatures (Celsius on Xaxis). Each compound has a different curve. S-C-9 -1_Solubility Presentation
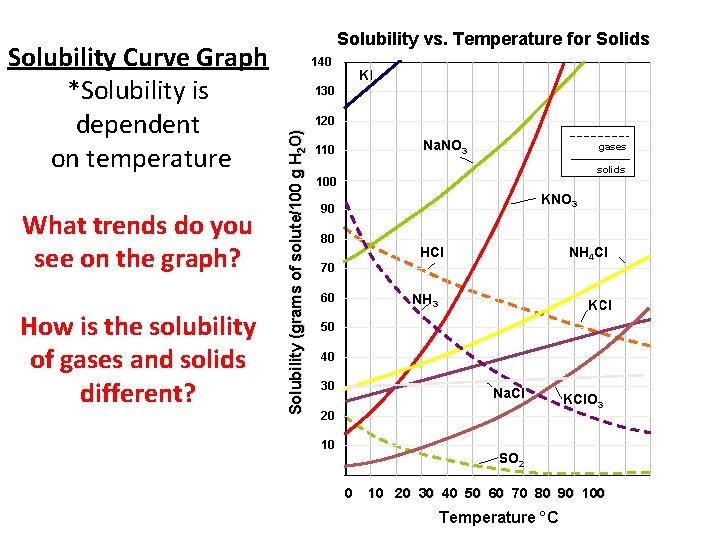
What trends do you see on the graph? How is the solubility of gases and solids different? 140 KI 130 120 Solubility (grams of solute/100 g H 2 O) Solubility Curve Graph *Solubility is dependent on temperature Solubility vs. Temperature for Solids Na. NO 3 110 gases solids 100 KNO 3 90 80 NH 4 Cl HCl 70 60 NH 3 KCl 50 40 30 Na. Cl 20 10 KCl. O 3 SO 2 0 10 20 30 40 50 60 70 80 90 100 Temperature °C
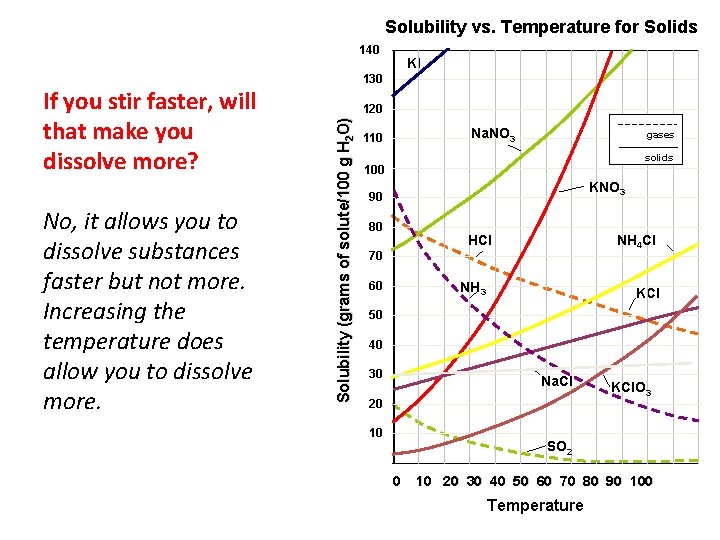
Solubility vs. Temperature for Solids 140 No, it allows you to dissolve substances faster but not more. Increasing the temperature does allow you to dissolve more. 130 120 Solubility (grams of solute/100 g H 2 O) If you stir faster, will that make you dissolve more? KI Na. NO 3 110 gases solids 100 KNO 3 90 80 NH 4 Cl HCl 70 60 NH 3 KCl 50 40 30 Na. Cl 20 10 KCl. O 3 SO 2 0 10 20 30 40 50 60 70 80 90 100 Temperature
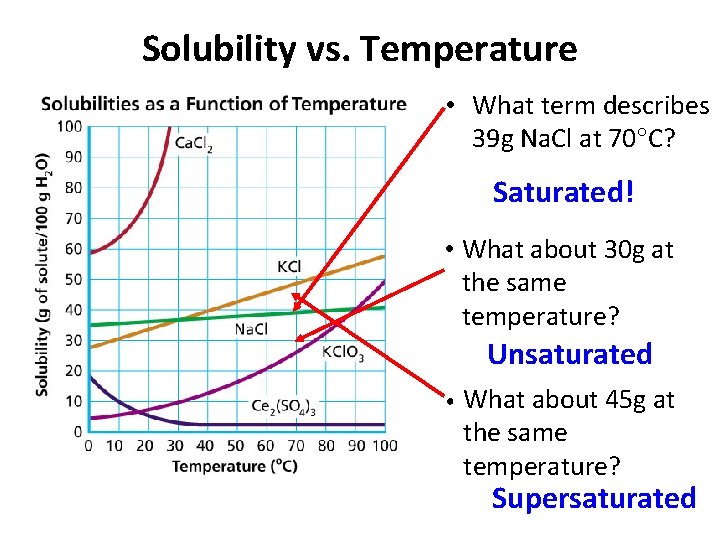
Solubility vs. Temperature • What term describes 39 g Na. Cl at 70 C? Saturated! • What about 30 g at the same temperature? Unsaturated • What about 45 g at the same temperature? Supersaturated
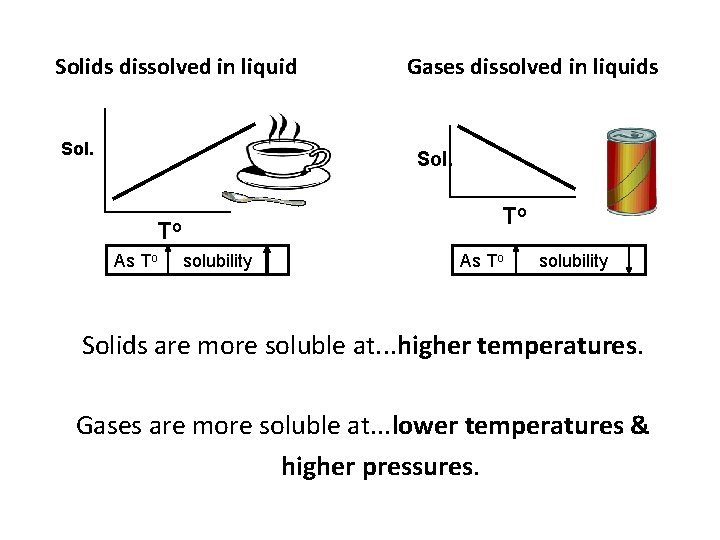
Solids dissolved in liquid Sol. Gases dissolved in liquids Sol. To To As To solubility Solids are more soluble at. . . higher temperatures. Gases are more soluble at. . . lower temperatures & higher pressures.

Pause for Solubility Practice Questions
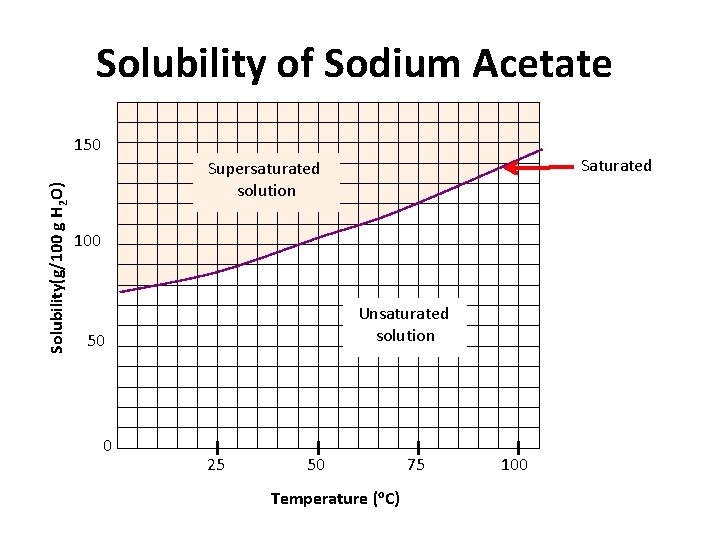
Solubility of Sodium Acetate Solubility(g/100 g H 2 O) 150 Saturated Supersaturated solution 100 Unsaturated solution 50 0 25 50 Temperature (o. C) 75 100
- Slides: 11