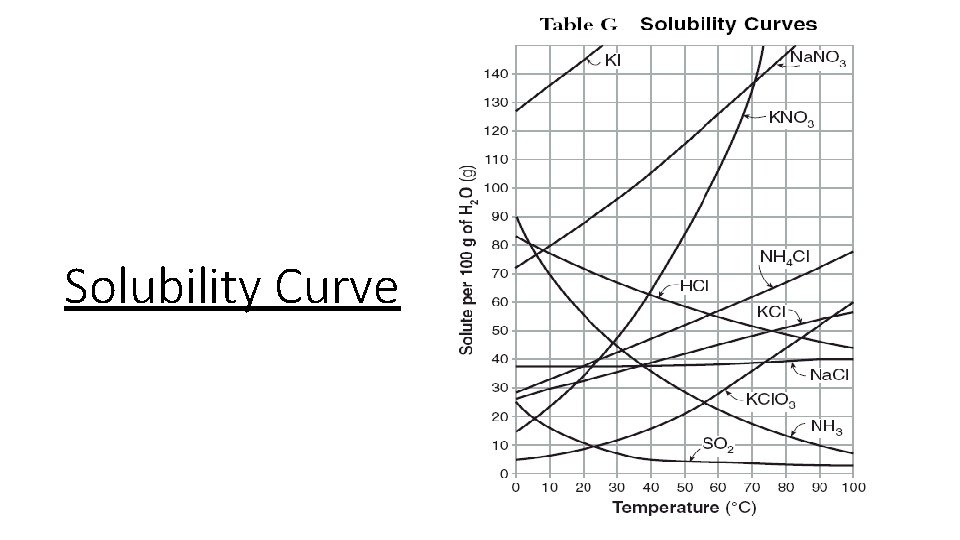
Solubility the concentration of a saturated solution at
Solubility: the concentration of a saturated solution at a specified temperature Units for solubility are simply units of concentration (% W/V, or mol/L) A temperature value has to accompany the solubility value!

Every pure substance has its own unique solubility – there is a maximum amount of solute that can be dissolved at a certain temperature before it no longer dissolves When you start to see a solid forming, you have hit the maximum amount of solute in a solution at that temperature (the solid is called a precipitate)


unsaturated: the amount of solute in the solution is less than the maximum amount saturated: the amount of solute in the solution is at the maximum amount supersaturated: the amount of solute in the solution is above the maximum amount

Solubility of Sodium Sulfate The solubility of sodium sulfate in water at 0ºC is 4. 76 g/100 m. L H 2 O. This means that 4. 76 grams of sodium sulfate can be dissolved in 100 m. L of water at 0ºC. If more than 4. 76 grams of sodium sulfate is added, it will not dissolve. Instead, it will start to form a crystallized precipitate from the excess solute. The quickest way to see if your solution is saturated or not is to look for the presence of undissolved solids in the solution.

Factors that affect solubility?

SOLIDS: • As the temperature increases, the solubility of solids in water increases as well. For example, the solubility of sucrose is 180 g/100 m. L at 0ºC and 487 g/100 m. L at 100ºC.
GASES: • As the temperature increases, the solubility of gases in water decreases. The kinetic energy increases as temperature increases. This means molecules move more and gases escape. • Pressures affects the solubility of gases: as pressure increases, the solubility of gases increases
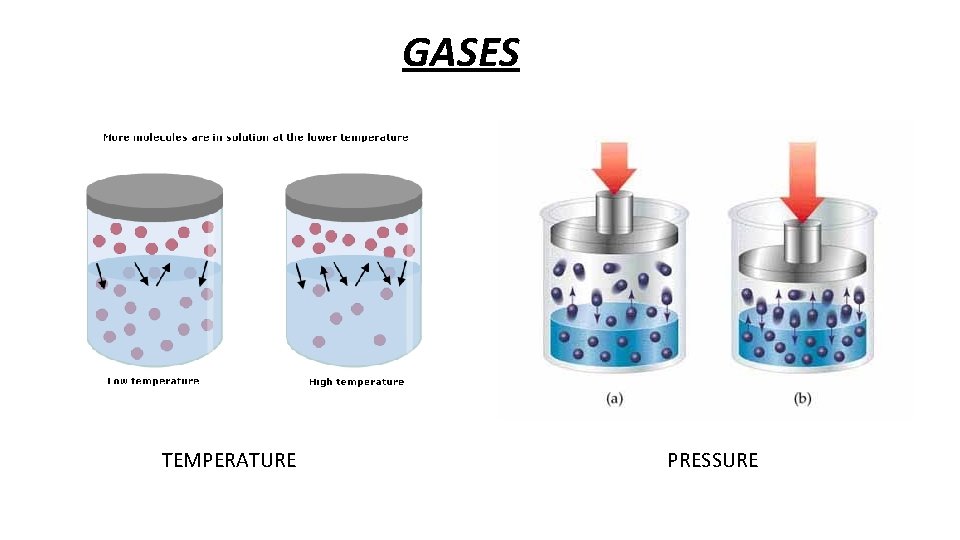
GASES TEMPERATURE PRESSURE

“The Bends” When divers are underwater, the pressure of their surroundings increases, which means the pressure causes divers to dissolve more air into their blood stream. As pressure decreases, solubility of gas decreases as well. As divers come back up, the nitrogen in the air starts to leave the bloodstream and cause nitrogen bubbles (which is painful). Nitrogen bubbles are very dangerous if they form in the brain or in the
LIQUIDS: It is difficult to generalize the effect of temperature on the solubility of liquids. However, for polar liquids, the solubility generally increases as the temperature increases. Some liquids (mostly nonpolar liquids) do not dissolve at all and form a separate layer instead. Ex) benzene, oil, gasoline – insoluble in water. We call these liquids immiscible. Some liquids (mostly polar liquids with hydrogen bonding capacity) dissolve in water no matter how much you add. Ex) ethanol, acetic acid in vinegar, antifreeze. We call these liquids miscible.

Oil + water Vinegar + water immiscible

Elements: Elements on their own typically have low solubility in water Ex) Carbon is used in many filtration systems to remove organic compounds that cause odours. The carbon does not dissolve in the water passing through it.
Solubility Curve
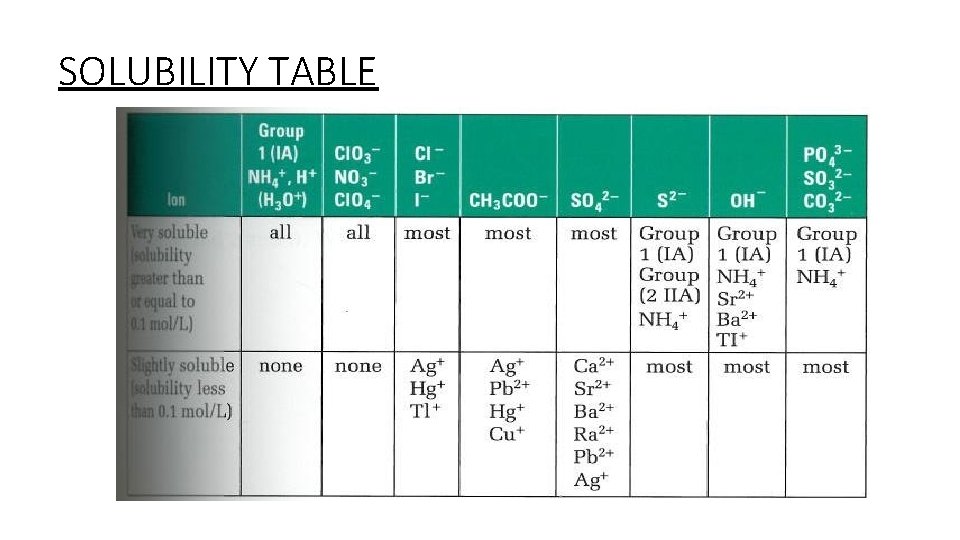
SOLUBILITY TABLE
- Slides: 15