Solubility Rules 1 2 3 4 5 6
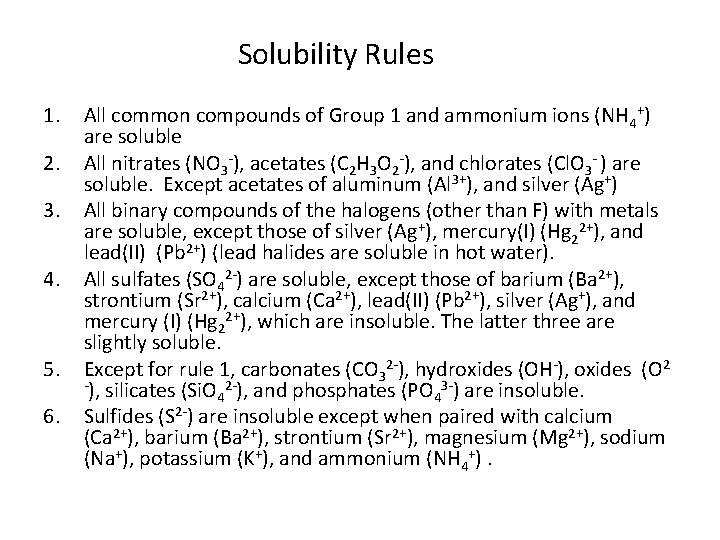
Solubility Rules 1. 2. 3. 4. 5. 6. All common compounds of Group 1 and ammonium ions (NH 4+) are soluble All nitrates (NO 3 -), acetates (C 2 H 3 O 2 -), and chlorates (Cl. O 3 - ) are soluble. Except acetates of aluminum (Al 3+), and silver (Ag+) All binary compounds of the halogens (other than F) with metals are soluble, except those of silver (Ag+), mercury(I) (Hg 22+), and lead(II) (Pb 2+) (lead halides are soluble in hot water). All sulfates (SO 42 -) are soluble, except those of barium (Ba 2+), strontium (Sr 2+), calcium (Ca 2+), lead(II) (Pb 2+), silver (Ag+), and mercury (I) (Hg 22+), which are insoluble. The latter three are slightly soluble. Except for rule 1, carbonates (CO 32 -), hydroxides (OH-), oxides (O 2 -), silicates (Si. O 2 -), and phosphates (PO 3 -) are insoluble. 4 4 Sulfides (S 2 -) are insoluble except when paired with calcium (Ca 2+), barium (Ba 2+), strontium (Sr 2+), magnesium (Mg 2+), sodium (Na+), potassium (K+), and ammonium (NH 4+).

active metals Metals Li K Ba Sr Ca Na Mg Al Mn Zn Cr Fe Cd Co Ni Sn Pb (H) Sb Cu Ag Pd Pt Hg Au Activity Series most reactive Will replace H 2 from liquid water, steam or acid Will replace H 2 from acid Will not replace H 2 from liquid water, steam or acid least reactive Nonmetals: Halogens F most reactive Cl Br I least reactive

Common Strong Acids and Bases Common Strong Acids Common Strong Bases Hydrochloric acid Hydrobromic acid Hydroiodic acid Perchloric acid Chloric acid Nitric acid Sulfuric acid Lithium hydroxide Li. OH (aq) Sodium hydroxide Potassium hydroxide Strontium hydroxide Barium hydroxide Calcium hydroxide Na. OH (aq) KOH (aq) Sr(OH)2 (aq) Ba(OH)2 (aq) Ca(OH)2 (aq) HCl (aq) HBr (aq) HI (aq) HCl. O 4 (aq) HCl. O 3 (aq) HNO 3 (aq) H 2 SO 4 (aq)
- Slides: 4