Solubility Notes Solutions SPS 6 Students will investigate

Solubility Notes
Solutions • SPS 6. Students will investigate the properties of solutions. • a. Describe solutions in terms of • solute/solvent • conductivity • concentration • b. Observe factors affecting the rate a solute dissolves in a specific solvent. • c. Demonstrate that solubility is related to temperature by constructing a solubility curve.
Solution • A mixture that is homogeneous on the molecular level. Example • Salt water • Gold jewelry • Cola
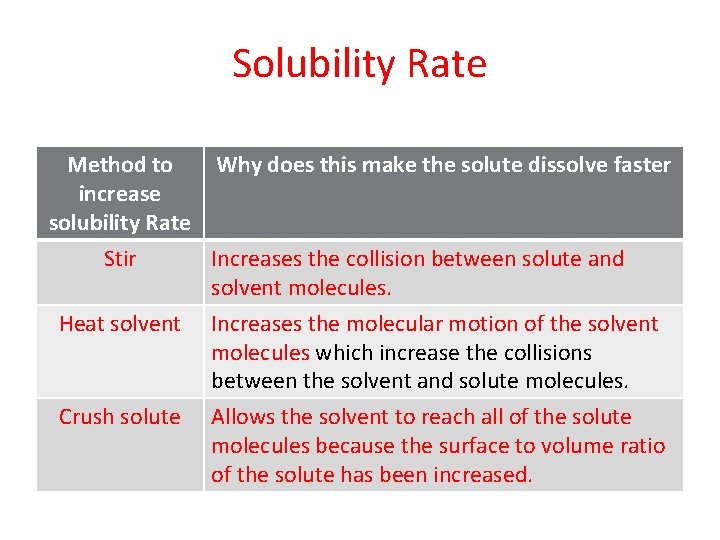
Solubility Rate Method to increase solubility Rate Stir Why does this make the solute dissolve faster Increases the collision between solute and solvent molecules. Heat solvent Increases the molecular motion of the solvent molecules which increase the collisions between the solvent and solute molecules. Crush solute Allows the solvent to reach all of the solute molecules because the surface to volume ratio of the solute has been increased.
Solute • The part that is dissolved in the solvent and is normally the smallest part of the solution. Example • Salt in salt water

Solvent • The part of the solution that does the dissolving, normally the largest part of a solution. Example • Water in salt water
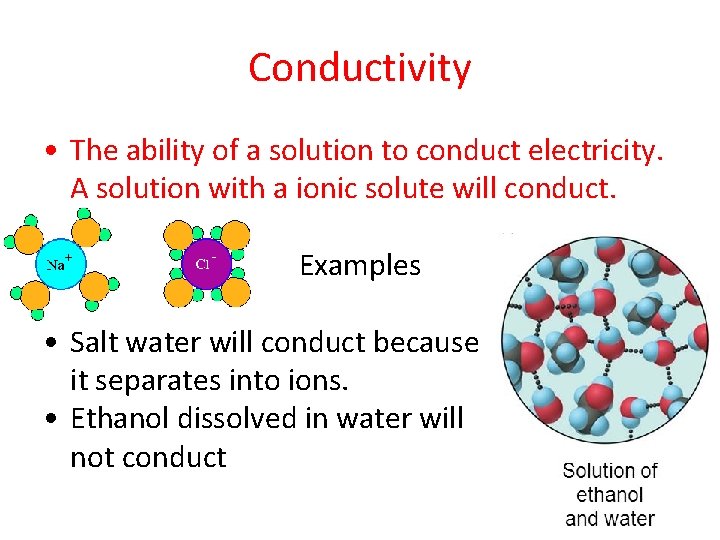
Conductivity • The ability of a solution to conduct electricity. A solution with a ionic solute will conduct. Examples • Salt water will conduct because it separates into ions. • Ethanol dissolved in water will not conduct
Concentration • A measure of how much solute is dissolved in the solvent. Example • How many grams of a solute is dissolved in 100 grams of water.

Saturated • A solution in which the maximum amount of solute has been dissolved. If more solute is added, it will sit as crystals on the bottom of the container. Example • Carbonated water • Sugar Kool-aid
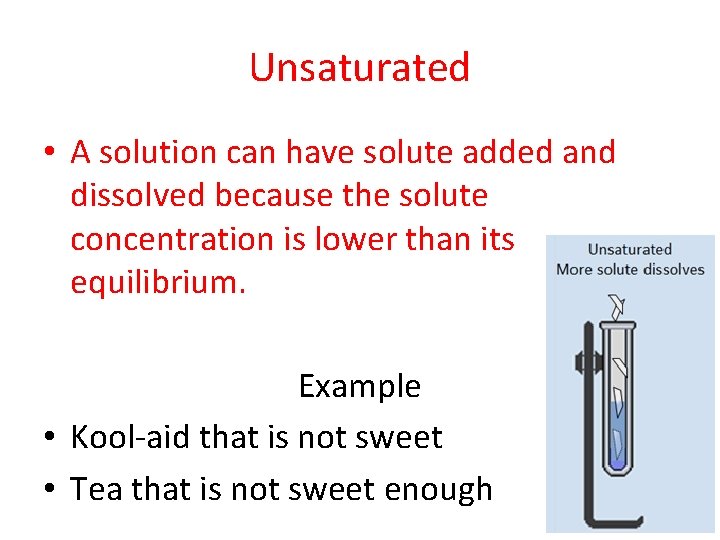
Unsaturated • A solution can have solute added and dissolved because the solute concentration is lower than its equilibrium. Example • Kool-aid that is not sweet • Tea that is not sweet enough
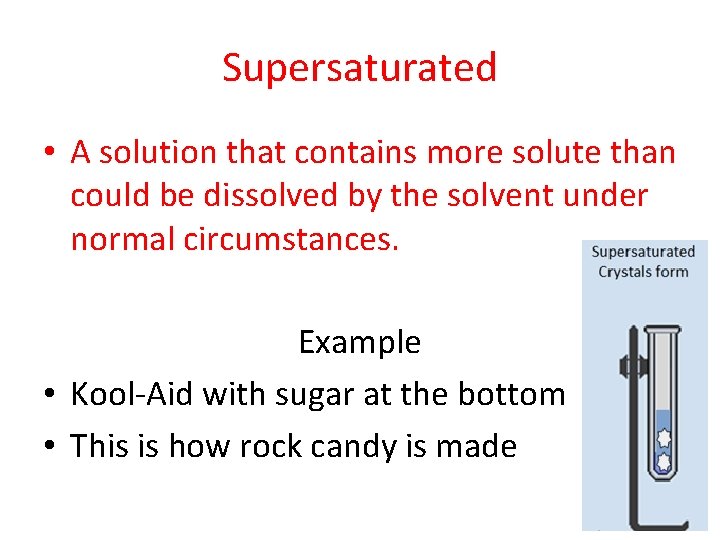
Supersaturated • A solution that contains more solute than could be dissolved by the solvent under normal circumstances. Example • Kool-Aid with sugar at the bottom • This is how rock candy is made
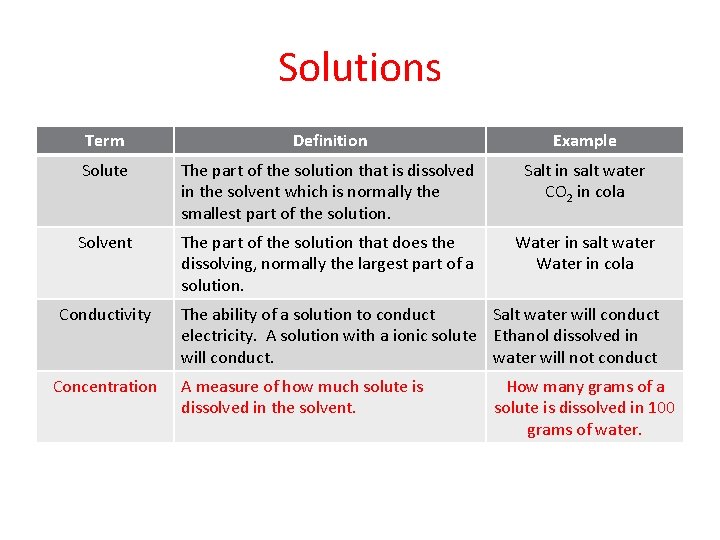
Solutions Term Definition Example Solute The part of the solution that is dissolved in the solvent which is normally the smallest part of the solution. Salt in salt water CO 2 in cola Solvent The part of the solution that does the dissolving, normally the largest part of a solution. Water in salt water Water in cola Conductivity Concentration The ability of a solution to conduct Salt water will conduct electricity. A solution with a ionic solute Ethanol dissolved in will conduct. water will not conduct A measure of how much solute is dissolved in the solvent. How many grams of a solute is dissolved in 100 grams of water.
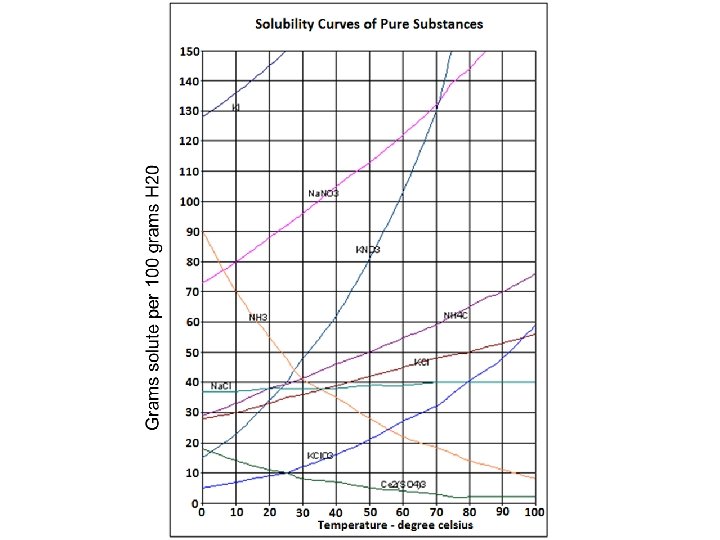
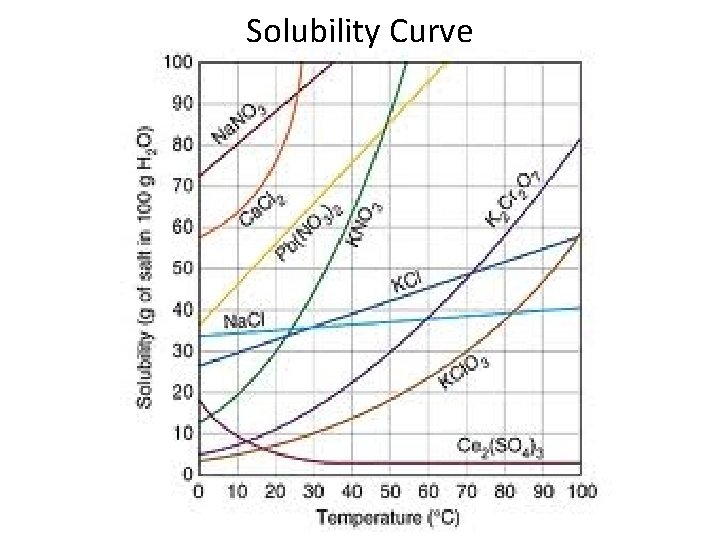
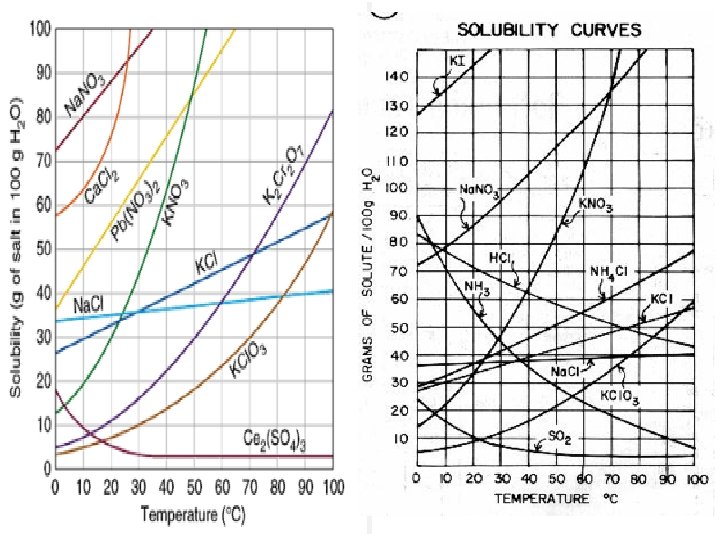
Solubility Curve
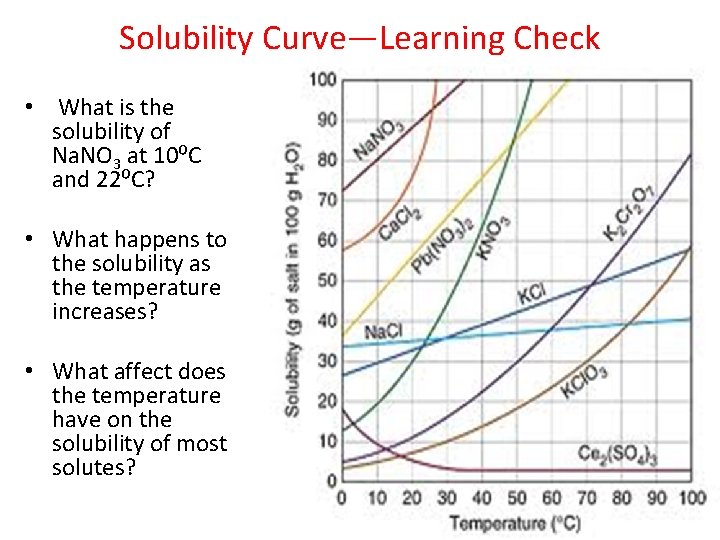
Solubility Curve—Learning Check • What is the solubility of Na. NO 3 at 10⁰C and 22⁰C? • What happens to the solubility as the temperature increases? • What affect does the temperature have on the solubility of most solutes?
- Slides: 16