Solubility Notes Solubility and Solubility Curves 1 Solubility

Solubility Notes Solubility and Solubility Curves 1

Solubility The solubility of a substance is determined by the mass of solute needed to make a saturated solution in 100 g (100 ml) of water at a certain temperature. Saturated – The total amount (mass) of solute that can be dissolved into solution at a certain temperature All points on the “solubility curve” Also known as the solution equilibrium line 2

Solubility Unsaturated: any point below the “solubility curve” of a substance at a certain temperature. Therefore, more solute can dissolve Supersaturated: any point above the “solubility curve” of a substance at a certain temperature Saturated: any point on the “solubility curve” 3
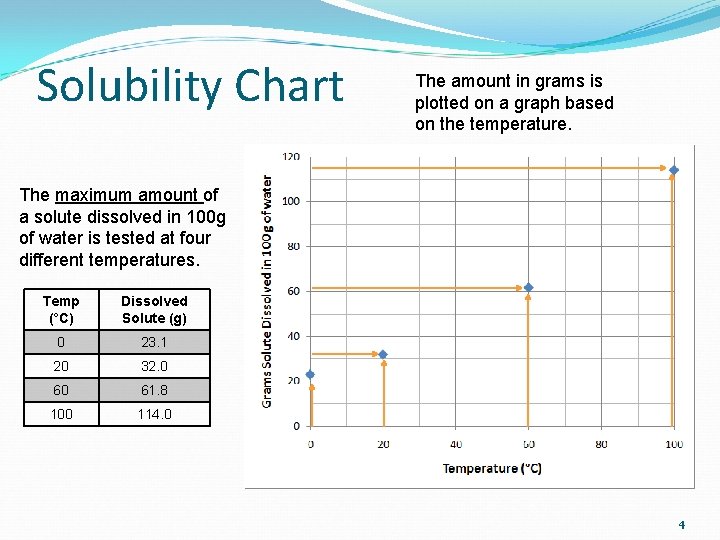
Solubility Chart The amount in grams is plotted on a graph based on the temperature. The maximum amount of a solute dissolved in 100 g of water is tested at four different temperatures. Temp (°C) Dissolved Solute (g) 0 23. 1 20 32. 0 60 61. 8 100 114. 0 4
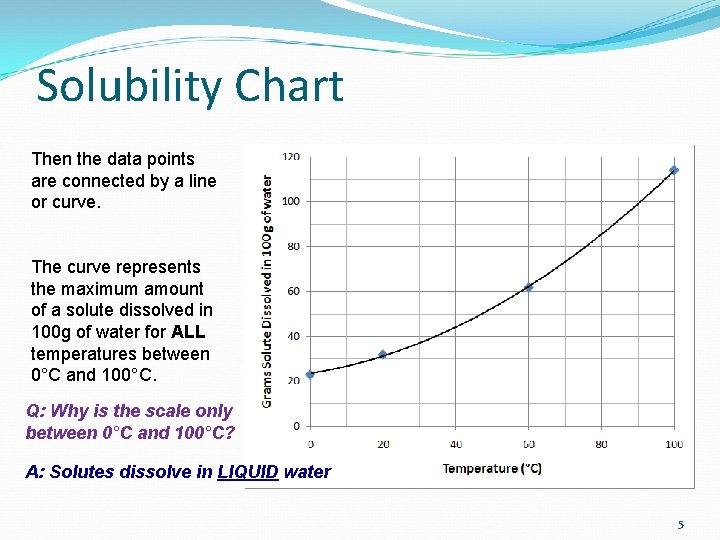
Solubility Chart Then the data points are connected by a line or curve. The curve represents the maximum amount of a solute dissolved in 100 g of water for ALL temperatures between 0°C and 100°C. Q: Why is the scale only between 0°C and 100°C? A: Solutes dissolve in LIQUID water 5
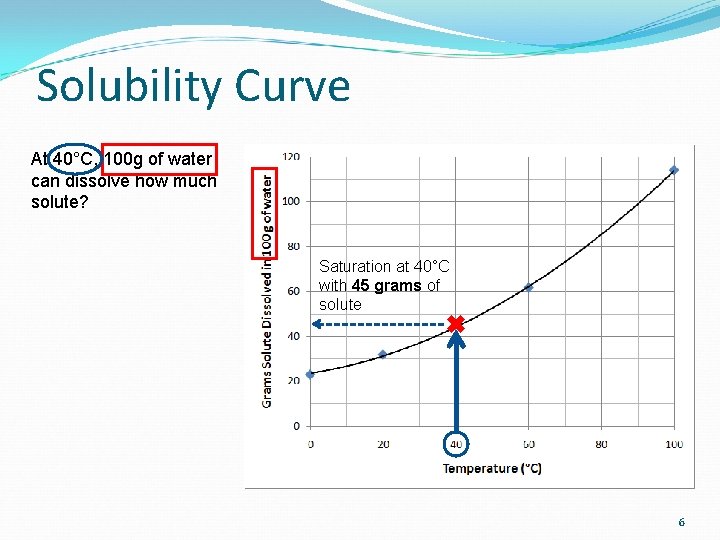
Solubility Curve At 40°C, 100 g of water can dissolve how much solute? Saturation at 40°C with 45 grams of solute 6
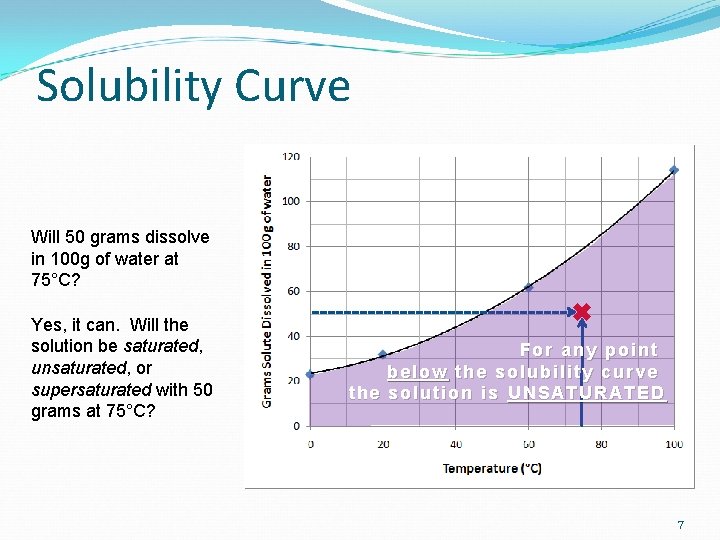
Solubility Curve Will 50 grams dissolve in 100 g of water at 75°C? Yes, it can. Will the solution be saturated, unsaturated, or supersaturated with 50 grams at 75°C? For any point below the solubility curve the solution is UNSATURATED 7
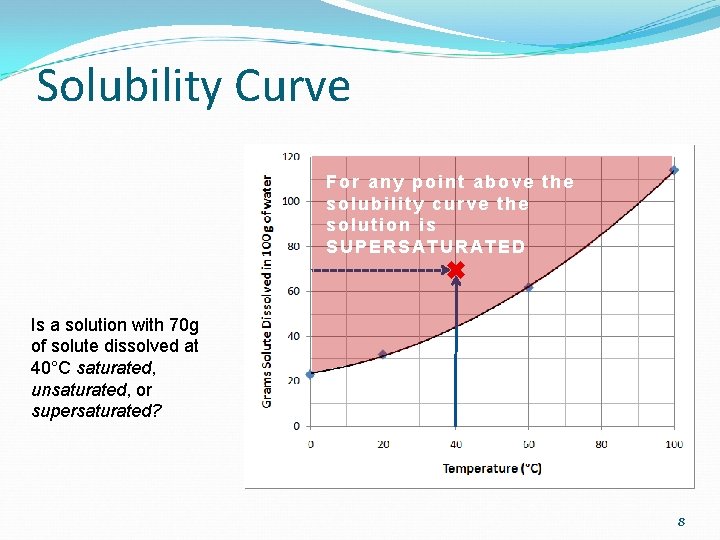
Solubility Curve For any point above the solubility curve the solution is SUPERSATURATED Is a solution with 70 g of solute dissolved at 40°C saturated, unsaturated, or supersaturated? 8

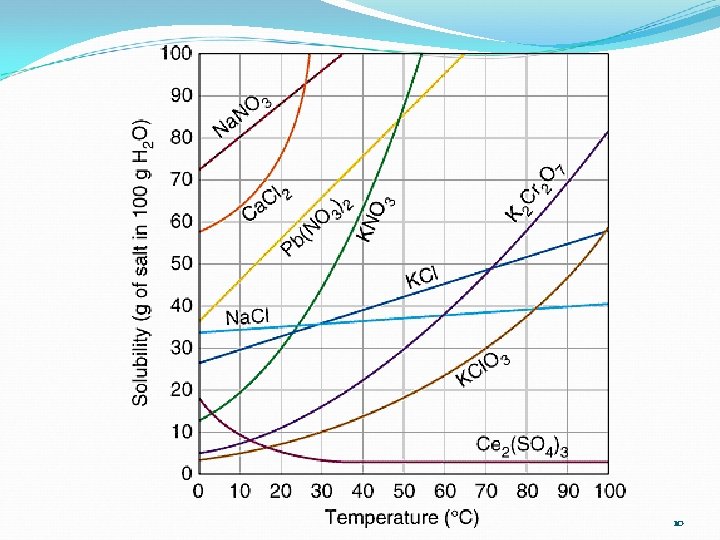
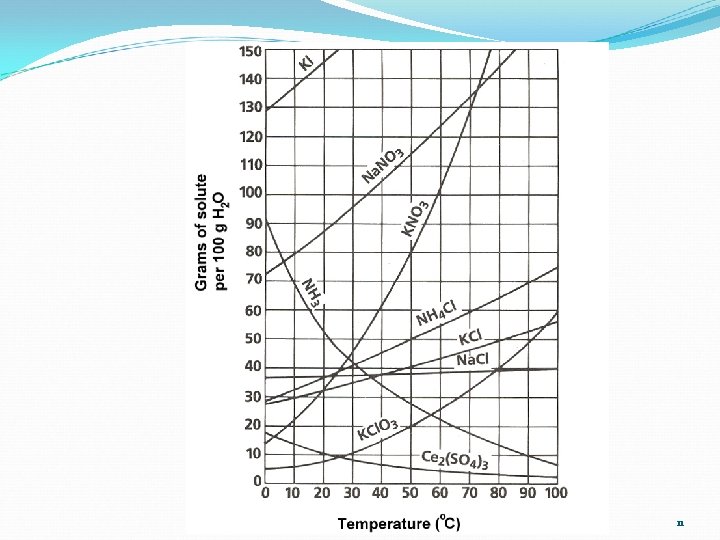
Solubility Questions 1. At 60°C, 38 g of solute is dissolved in 100 g of water. What is the name of the solute? 2. If 50 g of KCl are dissolved in 100 g of water, at 80°C, is the solution saturated, unsaturated, supersaturated? 3. What is the solubility of KNO 3 at 45°C in 200 g of water? 4. What is the solubility of KCl at 80°C in 150 g of water? 9
10
11
- Slides: 11