Solubility Lesson 3 Calculating Ksp The Molar Solubility

Solubility Lesson 3 Calculating Ksp
The Molar Solubility is the molarity required to saturate or fill the solution at any given temperature. 1. The solubility (s) of Ba. CO 3 is 5. 1 x 10 -5 M @ 250 C. Calculate the solubility product or Ksp.
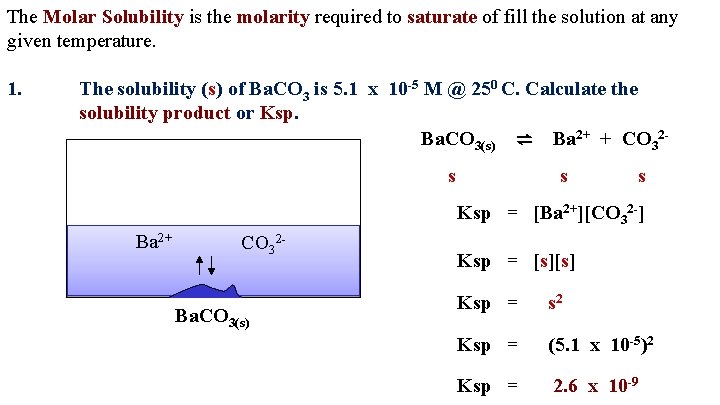
The Molar Solubility is the molarity required to saturate of fill the solution at any given temperature. 1. The solubility (s) of Ba. CO 3 is 5. 1 x 10 -5 M @ 250 C. Calculate the solubility product or Ksp. Ba. CO 3(s) ⇌ Ba 2+ + CO 32 s s s Ksp = [Ba 2+][CO 32 -] Ba 2+ CO 32 Ba. CO 3(s) Ksp = [s][s] Ksp = s 2 Ksp = (5. 1 x 10 -5)2 Ksp = 2. 6 x 10 -9

Ksp Solubility Product Saturated solutions- at equilibrium No Units Increasing Temperature increases the Ksp
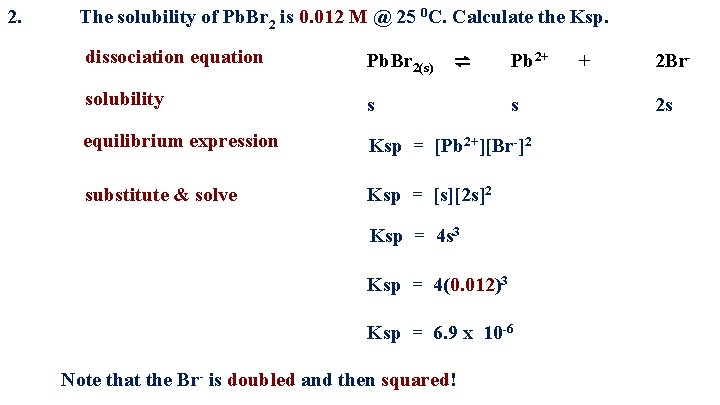
2. The solubility of Pb. Br 2 is 0. 012 M @ 25 0 C. Calculate the Ksp. dissociation equation Pb. Br 2(s) ⇌ Pb 2+ solubility s s equilibrium expression Ksp = [Pb 2+][Br-]2 substitute & solve Ksp = [s][2 s]2 Ksp = 4 s 3 Ksp = 4(0. 012)3 Ksp = 6. 9 x 10 -6 Note that the Br- is doubled and then squared! + 2 Br 2 s
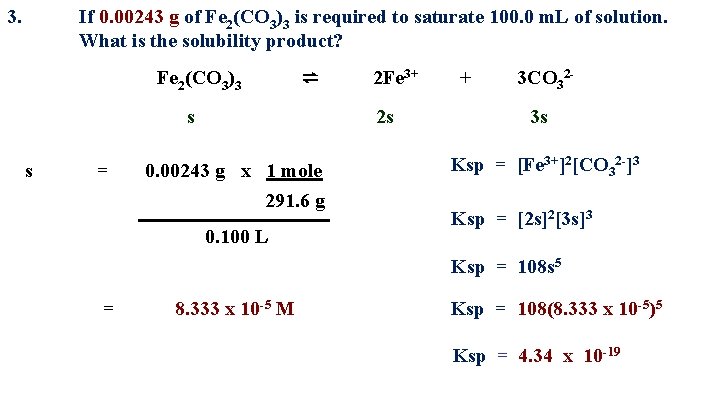
3. If 0. 00243 g of Fe 2(CO 3)3 is required to saturate 100. 0 m. L of solution. What is the solubility product? Fe 2(CO 3)3 ⇌ s s = 2 Fe 3+ 2 s 0. 00243 g x 1 mole 291. 6 g 0. 100 L + 3 CO 323 s Ksp = [Fe 3+]2[CO 32 -]3 Ksp = [2 s]2[3 s]3 Ksp = 108 s 5 = 8. 333 x 10 -5 M Ksp = 108(8. 333 x 10 -5)5 Ksp = 4. 34 x 10 -19
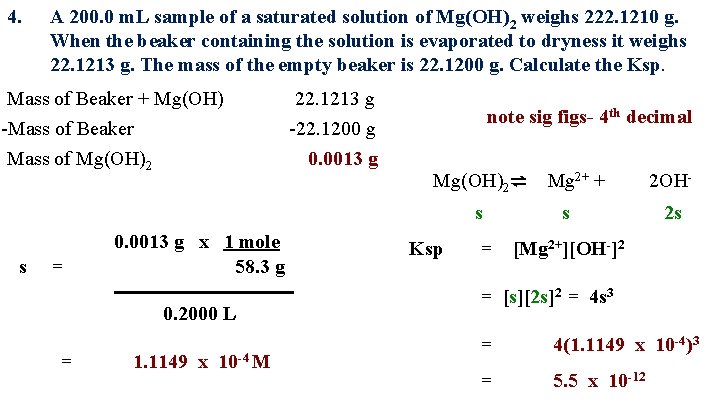
4. A 200. 0 m. L sample of a saturated solution of Mg(OH)2 weighs 222. 1210 g. When the beaker containing the solution is evaporated to dryness it weighs 22. 1213 g. The mass of the empty beaker is 22. 1200 g. Calculate the Ksp. Mass of Beaker + Mg(OH) -Mass of Beaker Mass of Mg(OH)2 22. 1213 g -22. 1200 g 0. 0013 g note sig figs- 4 th decimal Mg(OH)2⇌ Mg 2+ + s s = 0. 0013 g x 1 mole 58. 3 g 0. 2000 L = 1. 1149 x 10 -4 M Ksp = s 2 OH 2 s [Mg 2+][OH-]2 = [s][2 s]2 = 4 s 3 = 4(1. 1149 x 10 -4)3 = 5. 5 x 10 -12
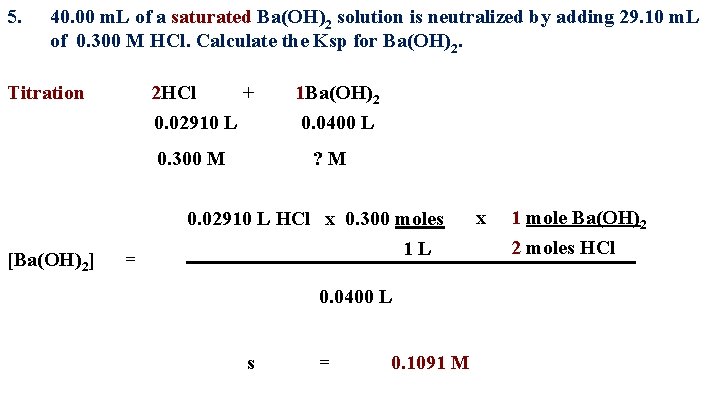
5. 40. 00 m. L of a saturated Ba(OH)2 solution is neutralized by adding 29. 10 m. L of 0. 300 M HCl. Calculate the Ksp for Ba(OH)2. Titration 2 HCl + 0. 02910 L 0. 300 M [Ba(OH)2] = 1 Ba(OH)2 0. 0400 L ? M 0. 02910 L HCl x 0. 300 moles 1 L 0. 0400 L s = 0. 1091 M x 1 mole Ba(OH)2 2 moles HCl
![Ksp ⇌ Ba(OH)2 s Ba 2+ s Ksp = [Ba 2+][OH-]2 = [s][2 s]2 Ksp ⇌ Ba(OH)2 s Ba 2+ s Ksp = [Ba 2+][OH-]2 = [s][2 s]2](http://slidetodoc.com/presentation_image_h2/ef43cdc47ff666ffe7c8ea3a2440cfce/image-9.jpg)
Ksp ⇌ Ba(OH)2 s Ba 2+ s Ksp = [Ba 2+][OH-]2 = [s][2 s]2 = 4 s 3 = 4(0. 1091)3 = 5. 20 x 10 -3 + 2 OH 2 s
- Slides: 9