Solubility Ksp Nuclear Ksp l Ksp the study
Solubility & Ksp & Nuclear
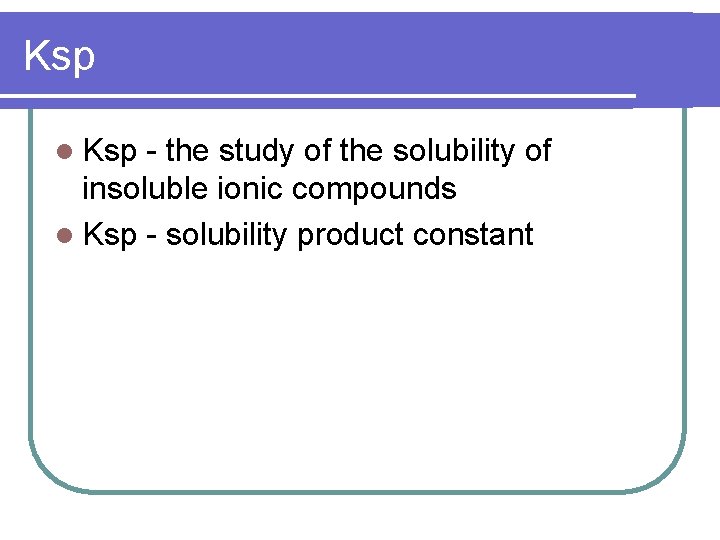
Ksp l Ksp - the study of the solubility of insoluble ionic compounds l Ksp - solubility product constant
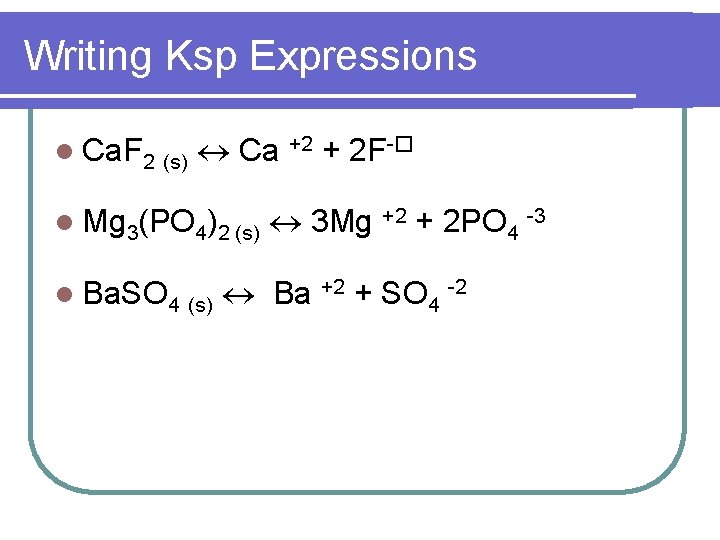
Writing Ksp Expressions l Ca. F 2 (s) Ca +2 + 2 F-� l Mg 3(PO 4)2 (s) l Ba. SO 4 (s) 3 Mg +2 + 2 PO 4 -3 Ba +2 + SO 4 -2
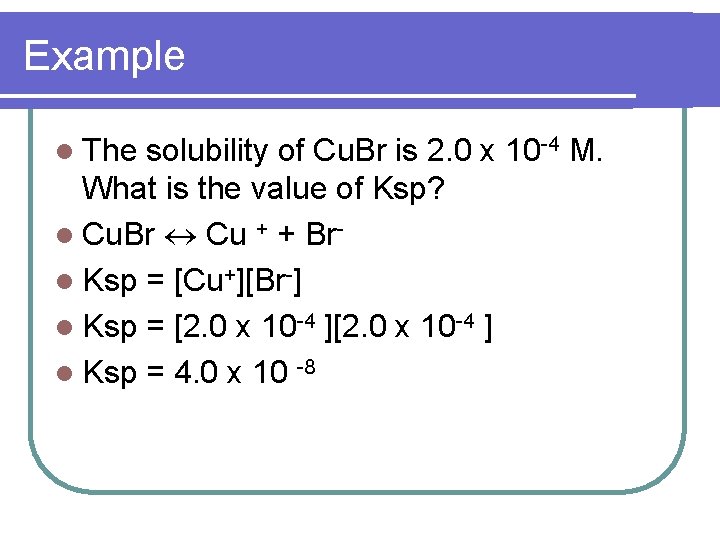
Example l The solubility of Cu. Br is 2. 0 x 10 -4 M. What is the value of Ksp? l Cu. Br Cu + + Brl Ksp = [Cu+][Br-] l Ksp = [2. 0 x 10 -4 ] l Ksp = 4. 0 x 10 -8
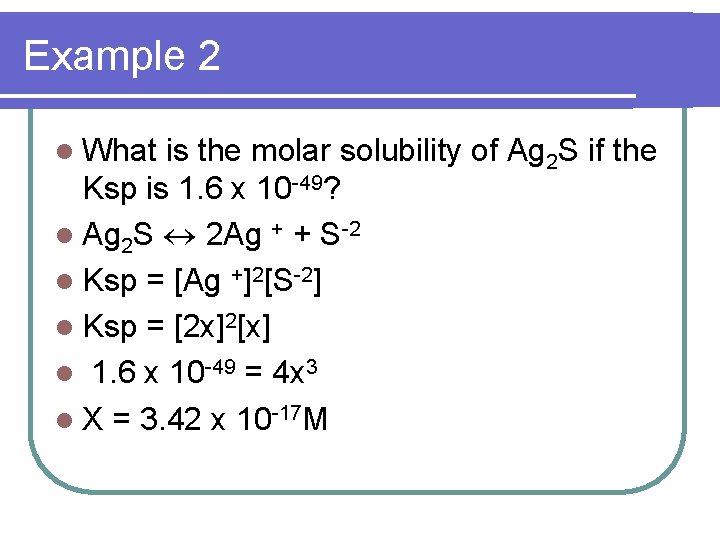
Example 2 l What is the molar solubility of Ag 2 S if the Ksp is 1. 6 x 10 -49? l Ag 2 S 2 Ag + + S-2 l Ksp = [Ag +]2[S-2] l Ksp = [2 x]2[x] l 1. 6 x 10 -49 = 4 x 3 l X = 3. 42 x 10 -17 M
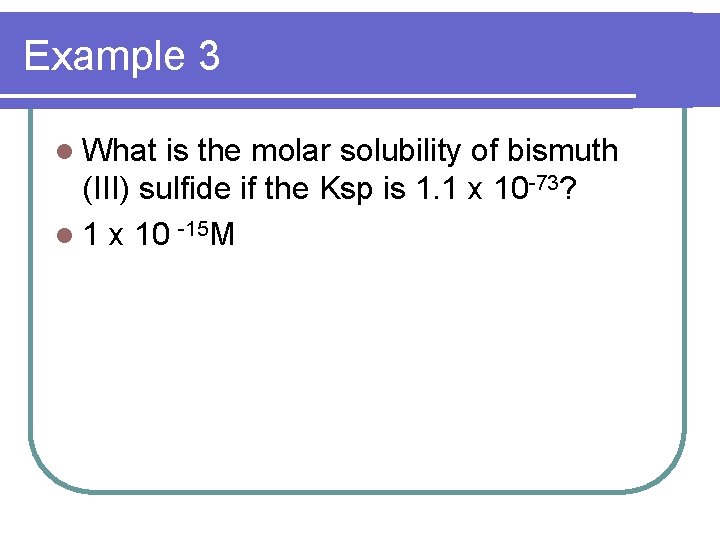
Example 3 l What is the molar solubility of bismuth (III) sulfide if the Ksp is 1. 1 x 10 -73? l 1 x 10 -15 M
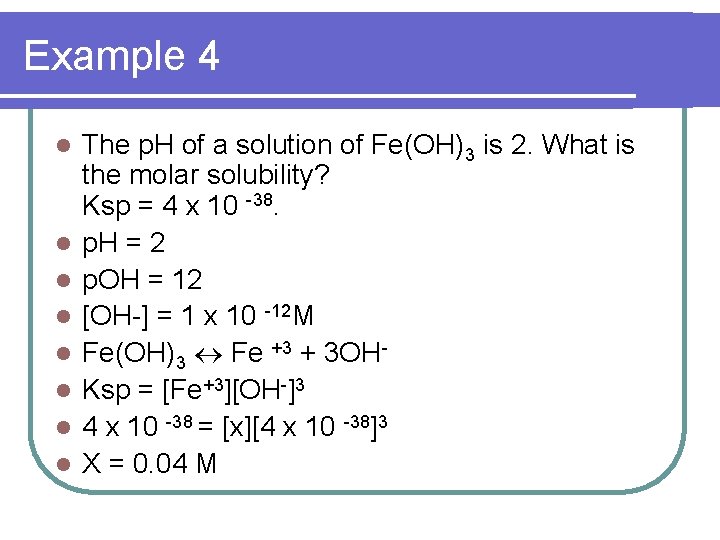
Example 4 l l l l The p. H of a solution of Fe(OH)3 is 2. What is the molar solubility? Ksp = 4 x 10 -38. p. H = 2 p. OH = 12 [OH-] = 1 x 10 -12 M Fe(OH)3 Fe +3 + 3 OHKsp = [Fe+3][OH-]3 4 x 10 -38 = [x][4 x 10 -38]3 X = 0. 04 M

Will a solid form? ? ? l Compare Ksp to Qsp l Q > K = yes ppt will form l Q < K = no ppt l Q is reaction quotient…just like K but not at equilibrium
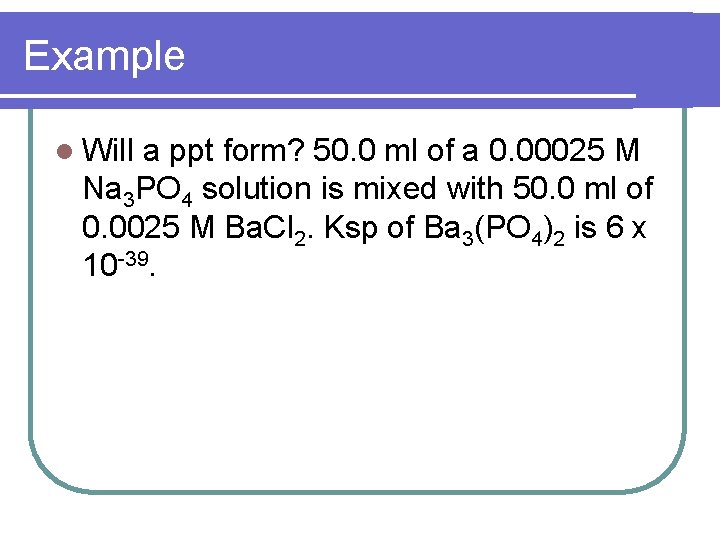
Example l Will a ppt form? 50. 0 ml of a 0. 00025 M Na 3 PO 4 solution is mixed with 50. 0 ml of 0. 0025 M Ba. Cl 2. Ksp of Ba 3(PO 4)2 is 6 x 10 -39.

Competing ppt l Na. Cl is added to a 50 ml beaker that contains a mixture of 0. 00015 M Pb(NO 3)2 and 0. 00035 M Ag. NO 3. What ppt will form 1 st? Show your work.
Nuclear Chemistry
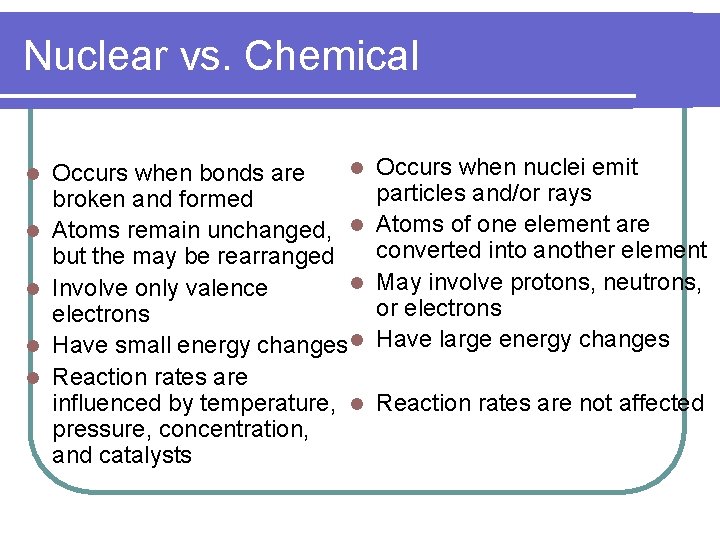
Nuclear vs. Chemical l l l Occurs when bonds are broken and formed Atoms remain unchanged, l but the may be rearranged l Involve only valence electrons Have small energy changes l Reaction rates are influenced by temperature, l pressure, concentration, and catalysts Occurs when nuclei emit particles and/or rays Atoms of one element are converted into another element May involve protons, neutrons, or electrons Have large energy changes Reaction rates are not affected
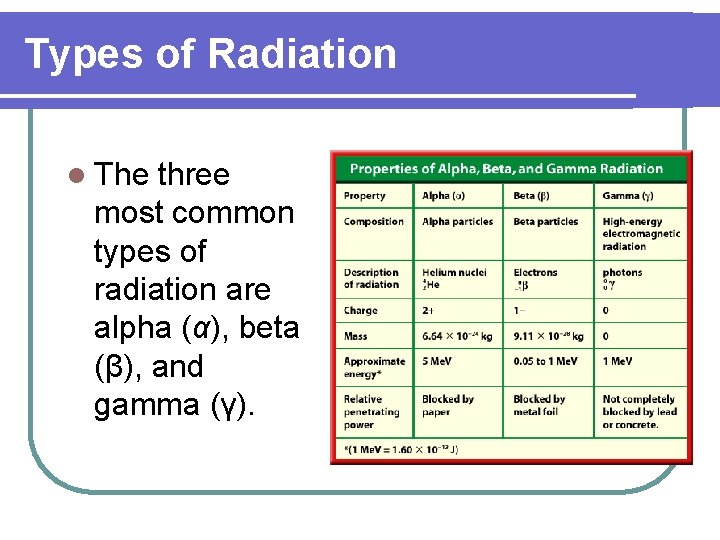
Types of Radiation l The three most common types of radiation are alpha (α), beta (β), and gamma (γ).
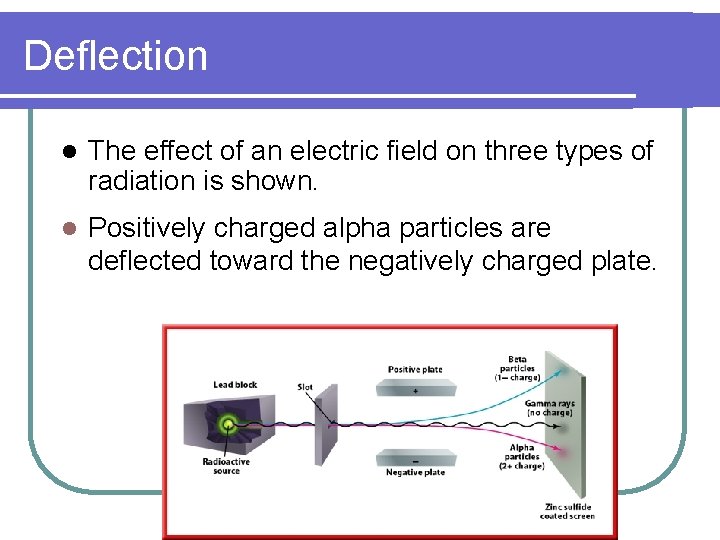
Deflection l The effect of an electric field on three types of radiation is shown. l Positively charged alpha particles are deflected toward the negatively charged plate.
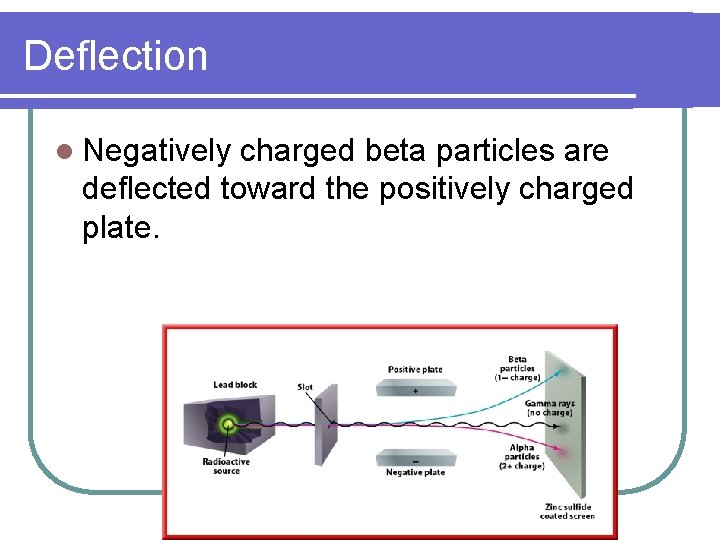
Deflection l Negatively charged beta particles are deflected toward the positively charged plate.
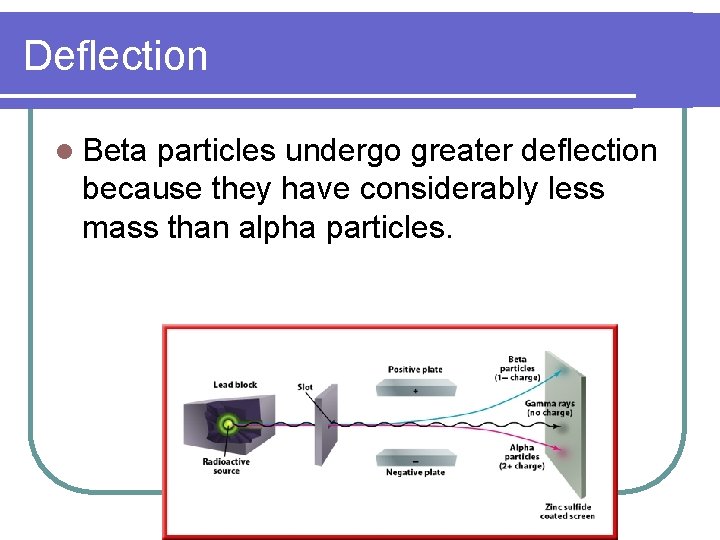
Deflection l Beta particles undergo greater deflection because they have considerably less mass than alpha particles.
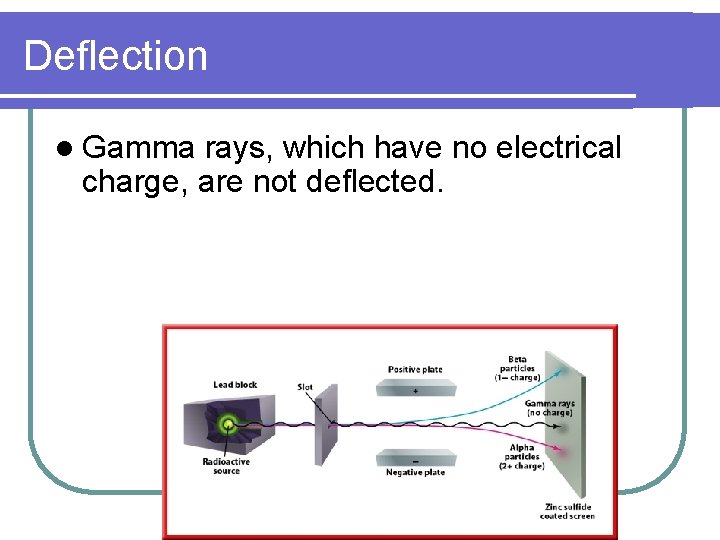
Deflection l Gamma rays, which have no electrical charge, are not deflected.

Writing Nuclear Reactions l When writing nuclear reactions, you must remember the Law of Conservation of Matter l What you start with has to equal what you end with l You also have to remember how to write formulas for isotopes
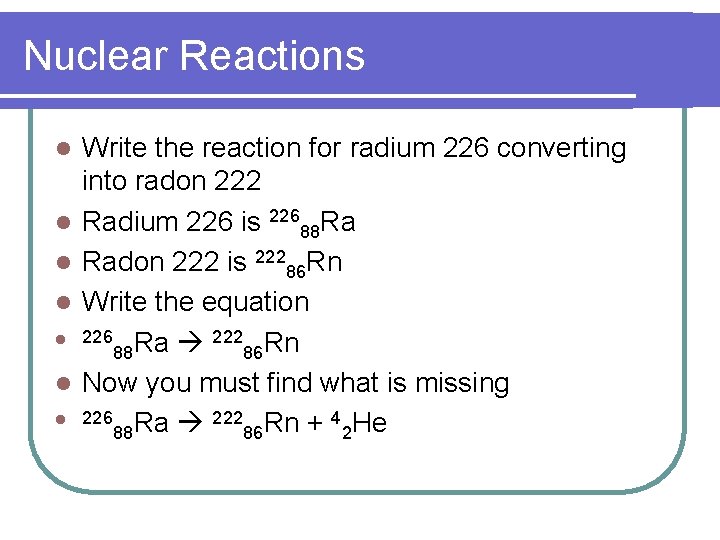
Nuclear Reactions l l l l Write the reaction for radium 226 converting into radon 222 Radium 226 is 22688 Ra Radon 222 is 22286 Rn Write the equation 226 Ra 222 Rn 88 86 Now you must find what is missing 226 Ra 222 Rn + 4 He 88 86 2
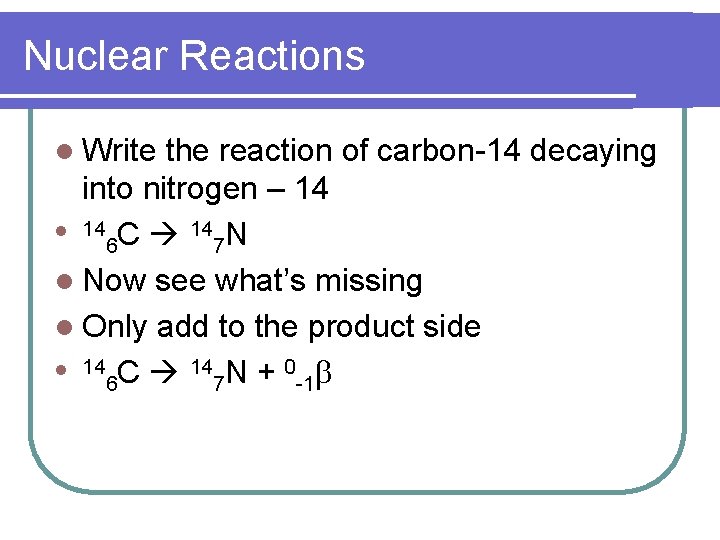
Nuclear Reactions l Write the reaction of carbon-14 decaying into nitrogen – 14 l 14 C 14 N 6 7 l Now see what’s missing l Only add to the product side l 14 C 14 N + 0 6 7 -1

Nuclear Reactions l Write the reaction of uranim-238 undergoing alpha and gamma decay l 238 U 4 He + 0 92 2 0 l Now put what’s left l 238 U 4 He + 0 + 234 Th 92 2 0 90
Fission and Fusion l Fission – splitting the nucleus into fragments l Releases large amounts of energy l Nuclear power plants use fission to generate power
Fission and Fusion l Fusion – combining of atomic nuclei l Release large amounts of energy l Require extremely high temperatures l The lowest temperature possible is 40, 000 K l Know to occur on the sun
- Slides: 23