Solubility equilibrium Solubility Property of a solid liquid

Solubility equilibrium

Solubility • Property of a solid, liquid, or gaseous chemical substance (solute) to dissolve in a solid, liquid, or gaseous substance (solvent). • As a result homogenous mixture is created.

Solubility • Usually expressed as the maximum amount of a substance, which can be dissolved in a specific volume of the solvent, under defined pressure and temperature conditions. • In certain conditions metastable supersaturated solution can be created.
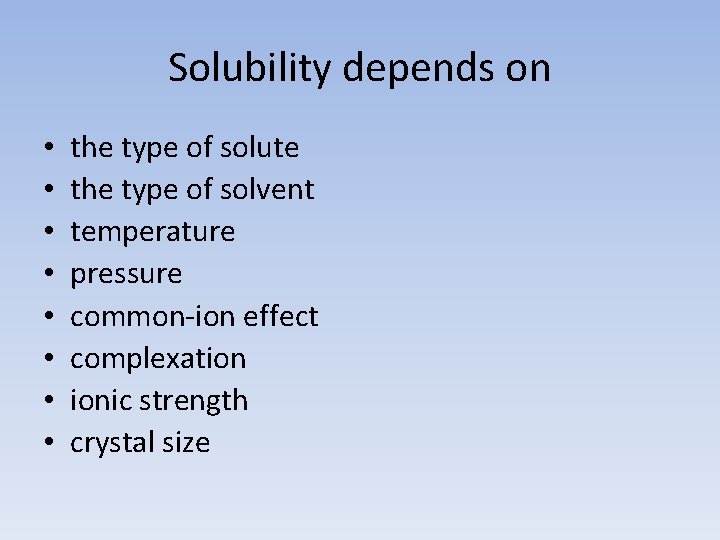
Solubility depends on • • the type of solute the type of solvent temperature pressure common-ion effect complexation ionic strength crystal size

Range Various substances solubility can vary in wide range. Substances sometimes are known as "insoluble", "poorly soluble", "highly soluble". Nowadays chemicals often takes the solubilities in range from 10 -12 kg/kg to complete miscibility with the solvent.

Sparingly soluble electrolyte • When the sparingly soluble electrolyte is put in water, only a small part goes into solution. • The transition electrolyte molecules to the liquid phase is called dissolution. It is associated with the molecules breakdown into ions: electrolytic dissociation. • Dissolution is often accompanied by the solvolysis and solvation processes.

Solvatation • Solvation is the process of surrounding solute with solvent. • Usually for highly polar solvents, especially when a soluble compound also has a strong polar properties or has ionic bonds. The solvation phenomenon is often a prelude to dissociation process.

Solvolysis • The chemical reaction between the chemical compound that is present in solution and the solvent • Solvolysis reactions are often assigned specific names derived from reactive solvent. • Water – hydrolysis, alcohol, - alcoholysis, acetic acid - acetolysis.
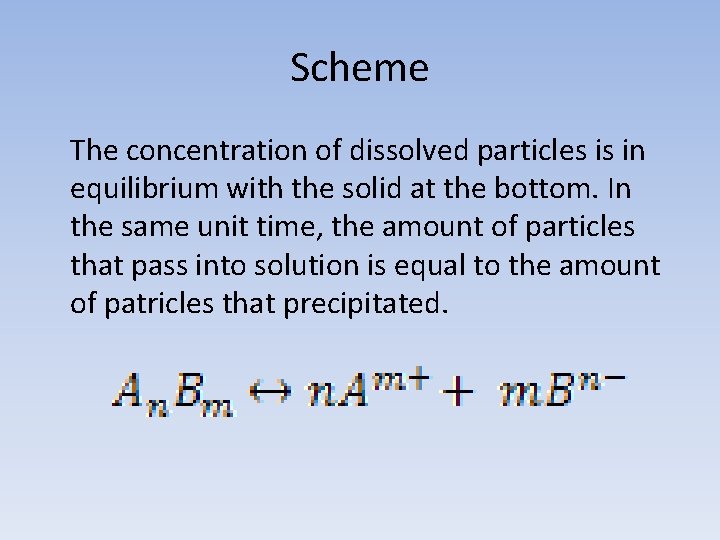
Scheme The concentration of dissolved particles is in equilibrium with the solid at the bottom. In the same unit time, the amount of particles that pass into solution is equal to the amount of patricles that precipitated.
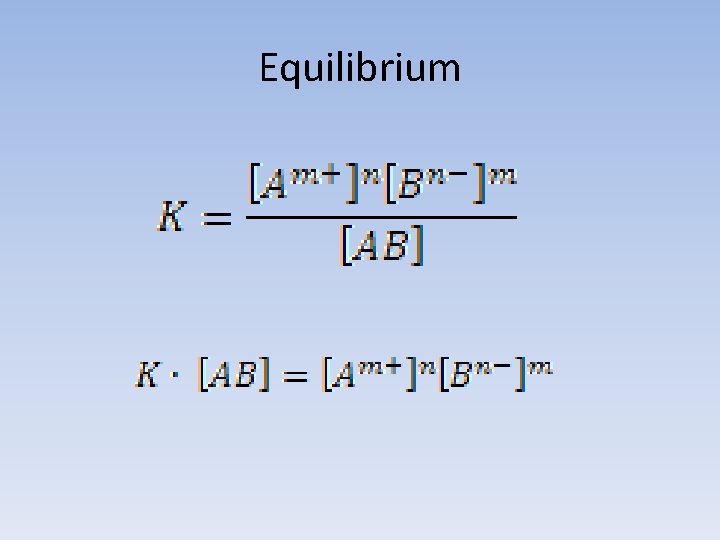
Equilibrium
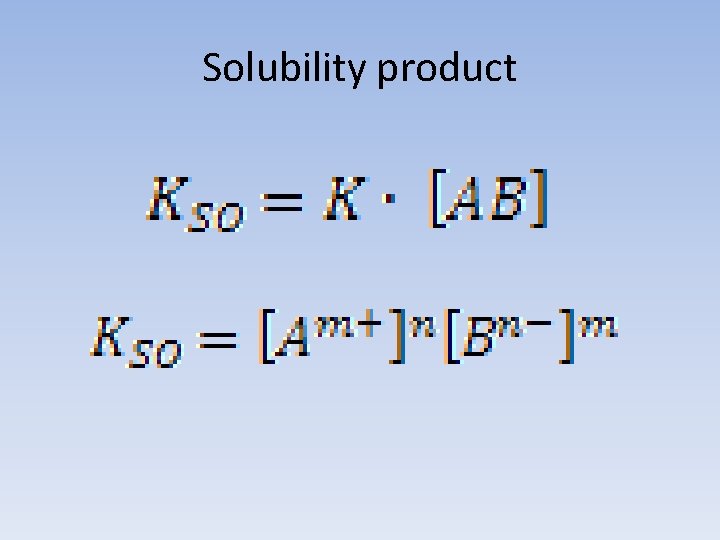
Solubility product

Solutions • Where the product of the ion concentrations is higher than the solubility product solution is supersaturated. • Where the product of the ion concentrations is equal to the solubility product solution is saturated. • Where the product of the ion concentrations is lower than the solubility product solution is unsaturated.

Usage Solubility product allows to calculate the concentration of saturated electrolyte solution
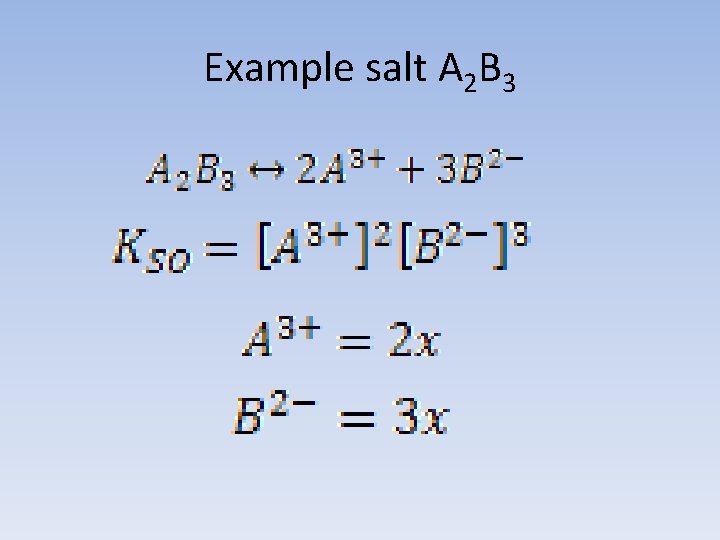
Example salt A 2 B 3
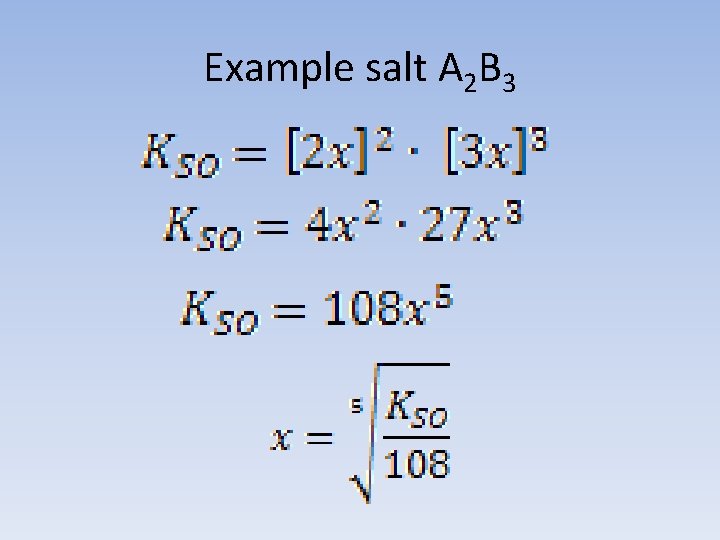
Example salt A 2 B 3

https: //www. boundless. com/chemistry/textbooks/boundless-chemistry-textbook/acid-base-equilibria-16/solubility-equilibria-120/solubility-product-487 -7263/images/solubility-product-constants-of-common-ions/

Example 1 Solubility of calcium carbonate in water at a temperature of 25°C, is 5*10 -9. What is the solubility of the salt, expressed in g/l under these conditions?
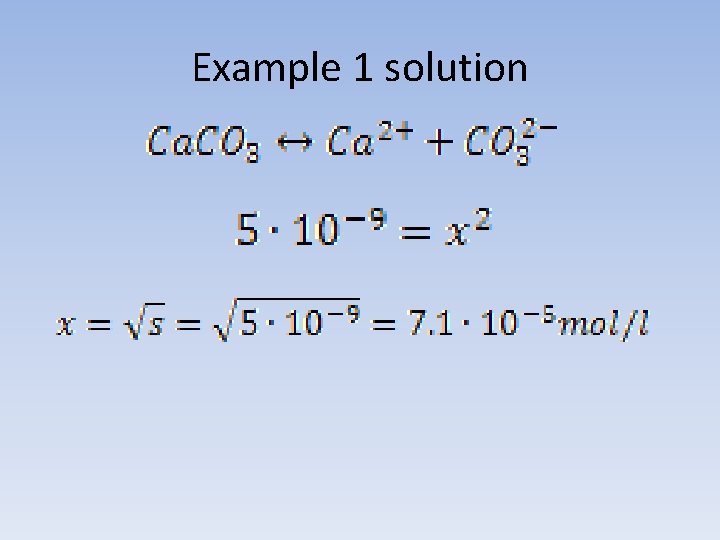
Example 1 solution
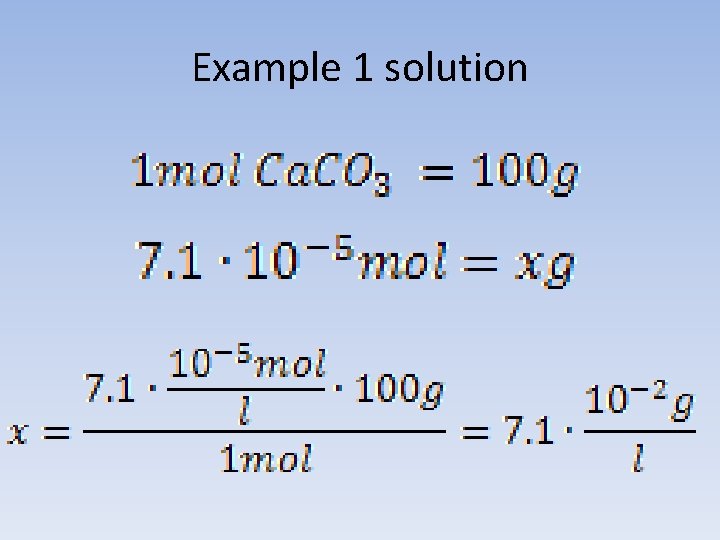
Example 1 solution

Example 2 Solubility product of silver sulfate (VI) in water, at a temperature 25°C is 7*10 -5. What is the solubility of the salt, expressed in g/l under these conditions?
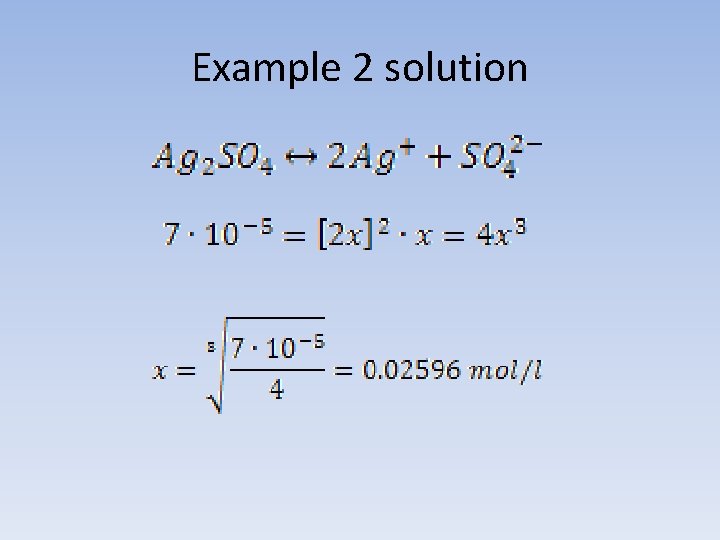
Example 2 solution
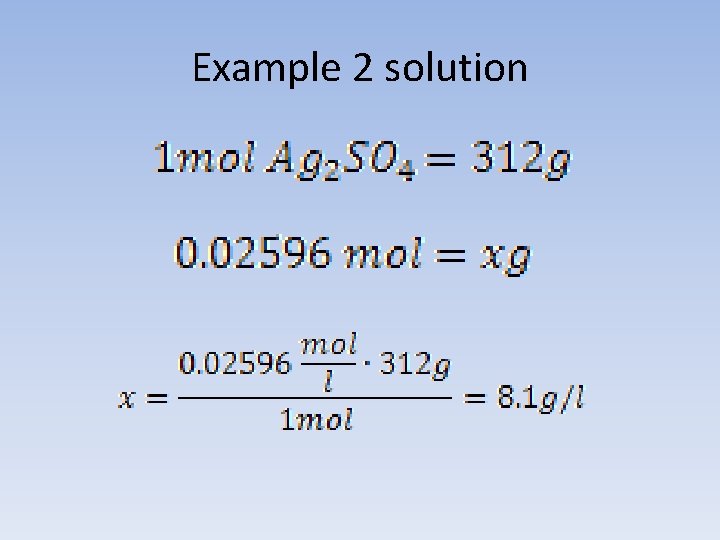
Example 2 solution

Example 3 The solubility of magnesium hydroxide at a temperature 180°C is equal to 2 × 104 mol/l. Find the solubility product of magnesium hydroxide.

Example 3 solution

Example 4 Lead iodide solubility product at room temperature is 1. 4 * 10 -8. Calculate solubility of the salt at the same temperature and the concentration of Pb 2+ and I- ion in the saturated solution.
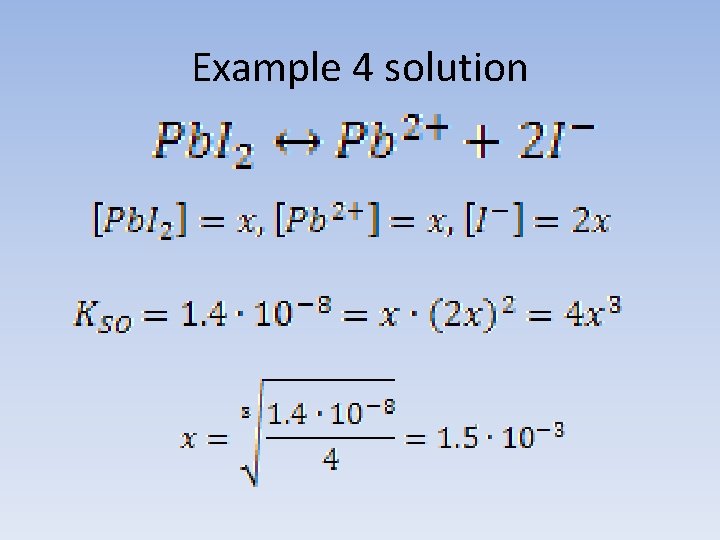
Example 4 solution

Example 5 Equal volumes of 0. 01 M solutions of calcium chloride and sodium sulfate were mixed. Does the calcium sulfate precipitate forms? The solubility product of calcium sulphate is equal to 2. 3 * 10 -4.
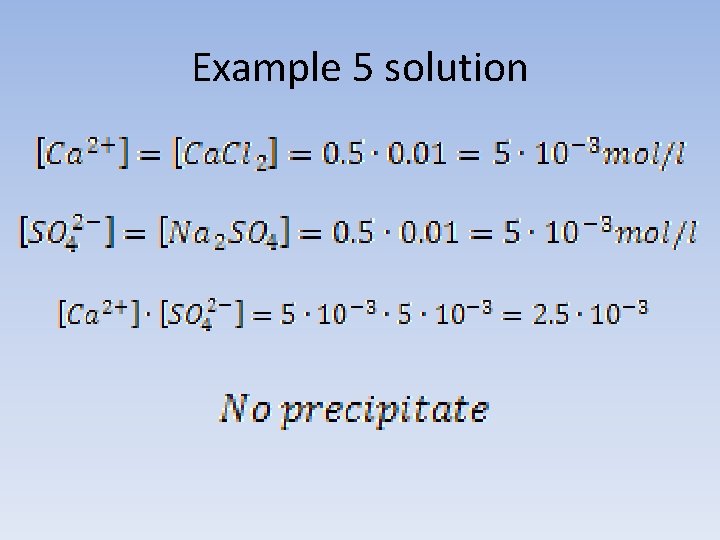
Example 5 solution

Bibliography and other sources • http: //chemed. chem. purdue. edu/genchem/to picreview/bp/ch 18/ksp. php • http: //bilbo. chm. uri. edu/CHM 112/tables/Ksp Table. htm
- Slides: 29