SOLUBILITY EQUILIBRIUM Ksp What is Ksp Solubility product

SOLUBILITY EQUILIBRIUM Ksp

What is Ksp? • Solubility product constant, last point of solubility • Generally used for solutions which would be identified as forming a precipitate • Ion-product expression: • Sr. F 2(s) ↔ Sr+2 (aq) + 2 F-(aq) • Ksp = [Sr+2][F-]2

Write the ion-product expression for each of the following: • a) magnesium carbonate • b) iron (II) hydroxide • C) calcium phosphate
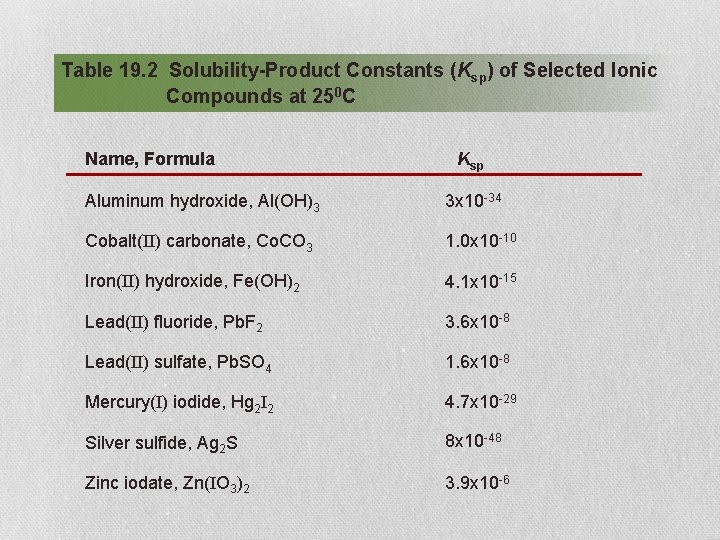
Table 19. 2 Solubility-Product Constants (Ksp) of Selected Ionic Compounds at 250 C Name, Formula Ksp Aluminum hydroxide, Al(OH)3 3 x 10 -34 Cobalt(II) carbonate, Co. CO 3 1. 0 x 10 -10 Iron(II) hydroxide, Fe(OH)2 4. 1 x 10 -15 Lead(II) fluoride, Pb. F 2 3. 6 x 10 -8 Lead(II) sulfate, Pb. SO 4 1. 6 x 10 -8 Mercury(I) iodide, Hg 2 I 2 4. 7 x 10 -29 Silver sulfide, Ag 2 S 8 x 10 -48 Zinc iodate, Zn(IO 3)2 3. 9 x 10 -6

Example 1 Calcium oxalate has a measured solubility of 4. 8 x 10 -5 M. Calculate Ksp value. • Write equation & Ksp expression. • Use ICE box format, plugin to expression & solve.

Example 2: Calculate solubility of silver phosphate if Ksp is 1. 8 x 10 -18 • Same set up, use unknown to solve for solubility.

• Hint: Change solubility to Molarity first! Example 3: Lead (II) sulfate is a key component in lead-acid batteries. Its solubility in water at 25˚C is 4. 25 x 10 -3 g/100 m. L solution. What is Ksp of Pb. SO 4?
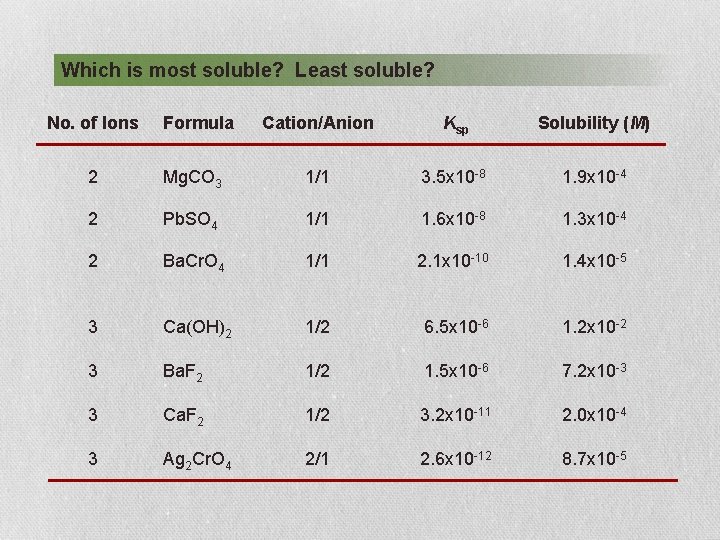
Which is most soluble? Least soluble? No. of Ions Formula Cation/Anion Ksp Solubility (M) 2 Mg. CO 3 1/1 3. 5 x 10 -8 1. 9 x 10 -4 2 Pb. SO 4 1/1 1. 6 x 10 -8 1. 3 x 10 -4 2 Ba. Cr. O 4 1/1 2. 1 x 10 -10 1. 4 x 10 -5 3 Ca(OH)2 1/2 6. 5 x 10 -6 1. 2 x 10 -2 3 Ba. F 2 1/2 1. 5 x 10 -6 7. 2 x 10 -3 3 Ca. F 2 1/2 3. 2 x 10 -11 2. 0 x 10 -4 3 Ag 2 Cr. O 4 2/1 2. 6 x 10 -12 8. 7 x 10 -5
- Slides: 8