Solubility Equilibria of Insoluble Ionic Compounds Concept Dissolution
Solubility Equilibria of “Insoluble” Ionic Compounds • Concept, Dissolution Equation and Ksp – And definition of “solubility” as “x” • Remember (relearn? ) your PAI’s and formulas of ionic compounds! *Read the blue – CHM 121 topic • Problem types (relate to prior types) – – Q vs K (becomes “Will a ppt form? ”) Plug-in (often in a “common ion” situation) Find K from [ ]0’s and one [ ]eq (Exp 26!) Find [ ]eq’s from [ ]0’s and K (“solubility”!) PS 05 -Ksp summary outine on this material! Has some examples, too. 1
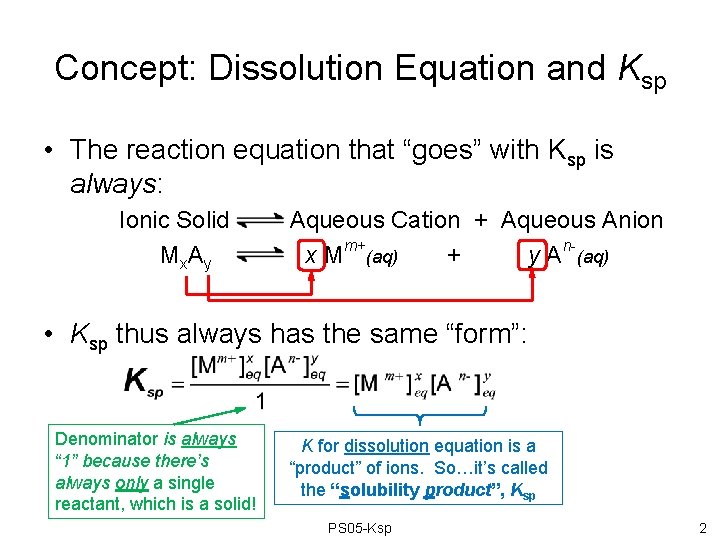
Concept: Dissolution Equation and Ksp • The reaction equation that “goes” with Ksp is always: Ionic Solid Mx. Ay Aqueous Cation + Aqueous Anion x Mm+(aq) + y An-(aq) • Ksp thus always has the same “form”: Denominator is always “ 1” because there’s always only a single reactant, which is a solid! K for dissolution equation is a “product” of ions. So…it’s called the “solubility product”, Ksp PS 05 -Ksp 2
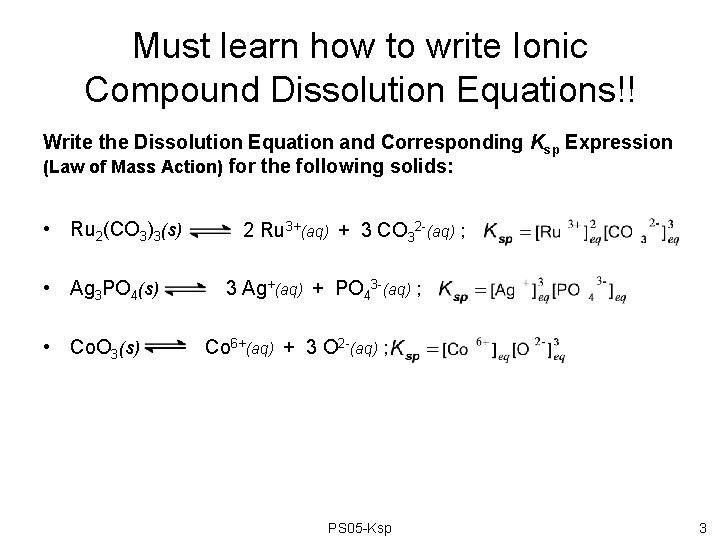
Must learn how to write Ionic Compound Dissolution Equations!! Write the Dissolution Equation and Corresponding Ksp Expression (Law of Mass Action) for the following solids: • Ru 2(CO 3)3(s) • Ag 3 PO 4(s) • Co. O 3(s) 2 Ru 3+(aq) + 3 CO 32 -(aq) ; 3 Ag+(aq) + PO 43 -(aq) ; Co 6+(aq) + 3 O 2 -(aq) ; PS 05 -Ksp 3
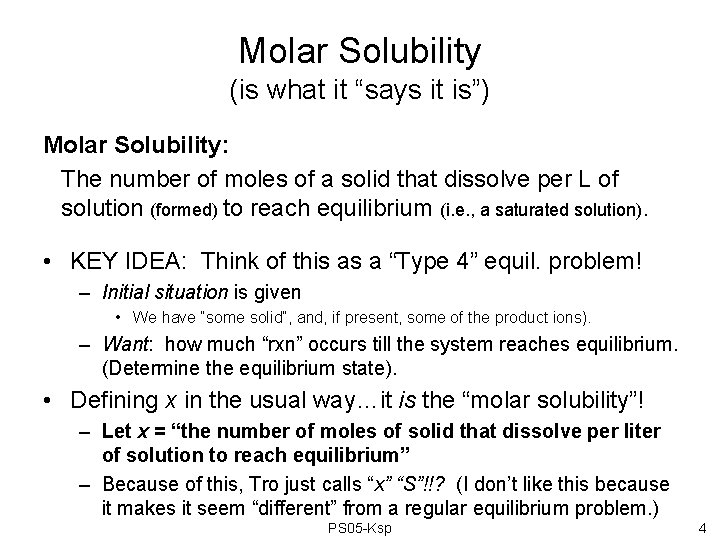
Molar Solubility (is what it “says it is”) Molar Solubility: The number of moles of a solid that dissolve per L of solution (formed) to reach equilibrium (i. e. , a saturated solution). • KEY IDEA: Think of this as a “Type 4” equil. problem! – Initial situation is given • We have “some solid”, and, if present, some of the product ions). – Want: how much “rxn” occurs till the system reaches equilibrium. (Determine the equilibrium state). • Defining x in the usual way…it is the “molar solubility”! – Let x = “the number of moles of solid that dissolve per liter of solution to reach equilibrium” – Because of this, Tro just calls “x” “S”!!? (I don’t like this because it makes it seem “different” from a regular equilibrium problem. ) PS 05 -Ksp 4
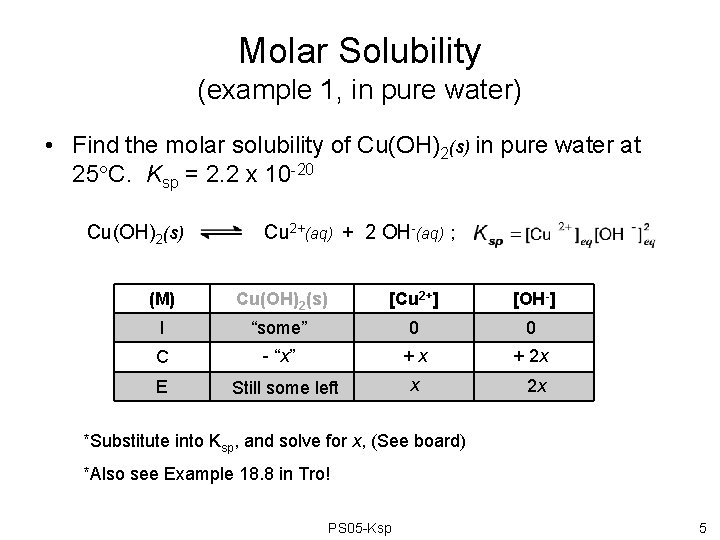
Molar Solubility (example 1, in pure water) • Find the molar solubility of Cu(OH)2(s) in pure water at 25 C. Ksp = 2. 2 x 10 -20 Cu(OH)2(s) Cu 2+(aq) + 2 OH-(aq) ; (M) Cu(OH)2(s) [Cu 2+] [OH-] I “some” 0 0 C - “x” +x + 2 x E Still some left x 2 x *Substitute into Ksp, and solve for x, (See board) *Also see Example 18. 8 in Tro! PS 05 -Ksp 5
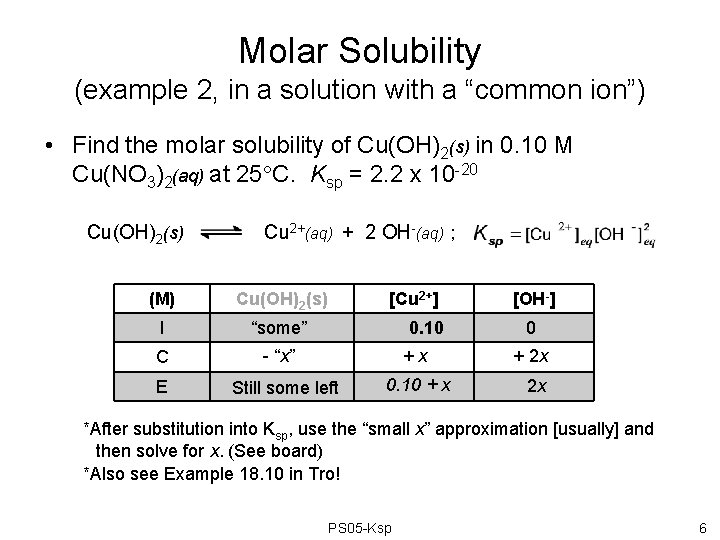
Molar Solubility (example 2, in a solution with a “common ion”) • Find the molar solubility of Cu(OH)2(s) in 0. 10 M Cu(NO 3)2(aq) at 25 C. Ksp = 2. 2 x 10 -20 Cu(OH)2(s) Cu 2+(aq) + 2 OH-(aq) ; (M) Cu(OH)2(s) I “some” C - “x” E [Cu 2+] 0. 10 +x Still some left 0. 10 + x [OH-] 0 + 2 x 2 x *After substitution into Ksp, use the “small x” approximation [usually] and then solve for x. (See board) *Also see Example 18. 10 in Tro! PS 05 -Ksp 6

Will a precipitate form? • For a Ksp type of equilibrium / problem, “precipitate forming” means “reverse reaction occurs”! • Since reverse rxn occurs only with Q > K, the question simply becomes: – Is Q > K ? [“Is Q > Ksp? ”] – See Example 18. 12 in Tro • NOTE: Must consider mixing, if applicable (see tan handout sheet for Exp 26 and). PS 05 -Ksp 7

“Type 3” Kind of Problem (Experiment 26!) • Know initial state info, and one [ion]eq, find K. • See tan sheet from Exp 26 PS 05 -Ksp 8
- Slides: 8