Solubility Dilution Molarity Molality Solutions Our next topic
Solubility, Dilution, Molarity, Molality

Solutions • Our next topic in chemistry is solutions, which are a type of mixture in which a solute has been dissolved by a solvent. • Today we are going to explore how to interpret and construct solubility curves. • We will learn what it means for a solution to be unsaturated, and supersaturated.

Solutions are composed of a SOLVENT and at least one SOLUTE. For salt water, salt is the solute and water is the solvent. Oceans are a SOLUTION of salt plus water SOLUTE = substance that dissolves DEMONSTRATION: Salt in water SOLVENT = substance into which the solute dissolves
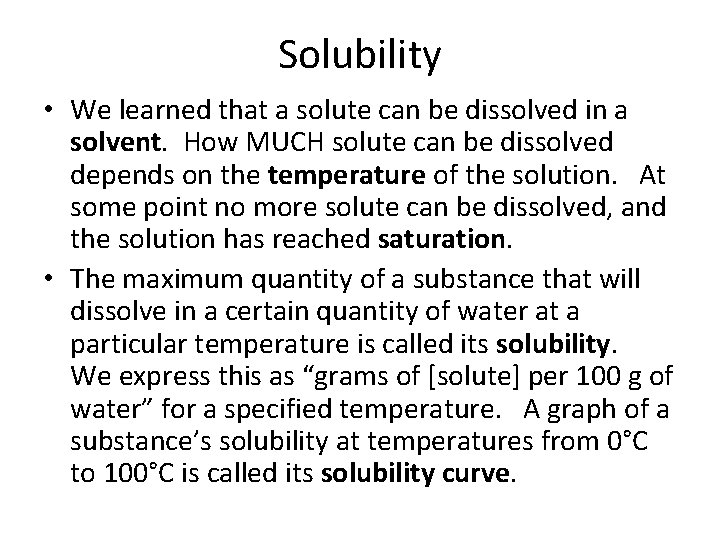
Solubility • We learned that a solute can be dissolved in a solvent. How MUCH solute can be dissolved depends on the temperature of the solution. At some point no more solute can be dissolved, and the solution has reached saturation. • The maximum quantity of a substance that will dissolve in a certain quantity of water at a particular temperature is called its solubility. We express this as “grams of [solute] per 100 g of water” for a specified temperature. A graph of a substance’s solubility at temperatures from 0°C to 100°C is called its solubility curve.
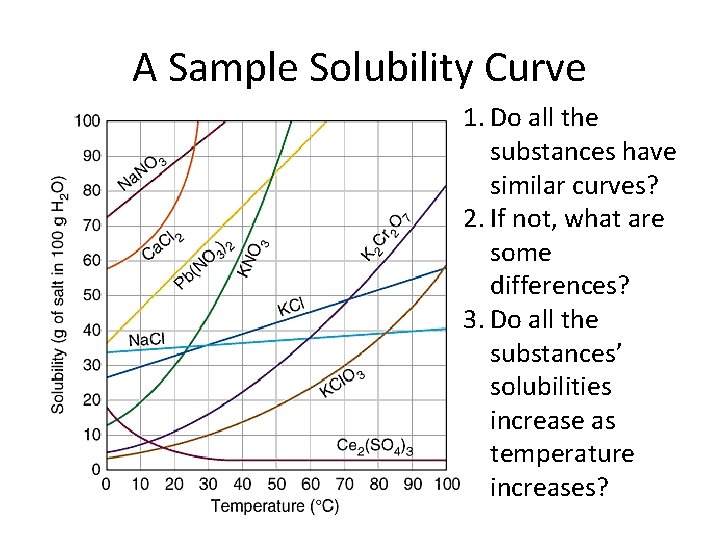
A Sample Solubility Curve 1. Do all the substances have similar curves? 2. If not, what are some differences? 3. Do all the substances’ solubilities increase as temperature increases?
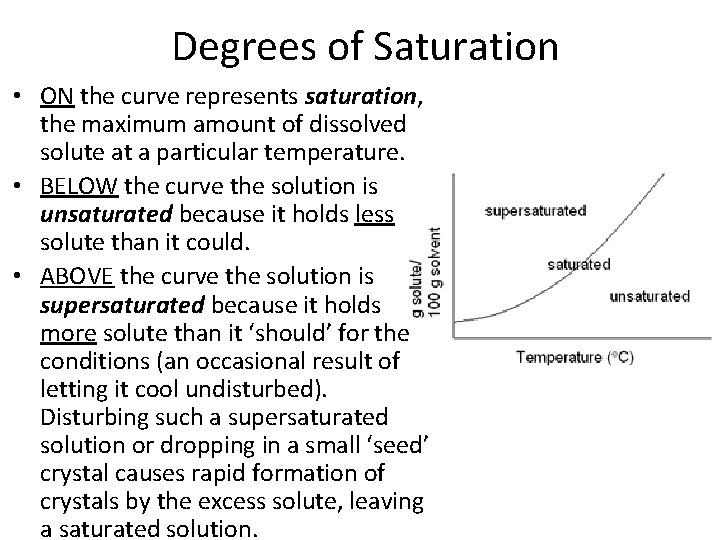
Degrees of Saturation • ON the curve represents saturation, the maximum amount of dissolved solute at a particular temperature. • BELOW the curve the solution is unsaturated because it holds less solute than it could. • ABOVE the curve the solution is supersaturated because it holds more solute than it ‘should’ for the conditions (an occasional result of letting it cool undisturbed). Disturbing such a supersaturated solution or dropping in a small ‘seed’ crystal causes rapid formation of crystals by the excess solute, leaving a saturated solution.
Solutions All SOLUTIONS are homogeneous mixtures of two or more substances.
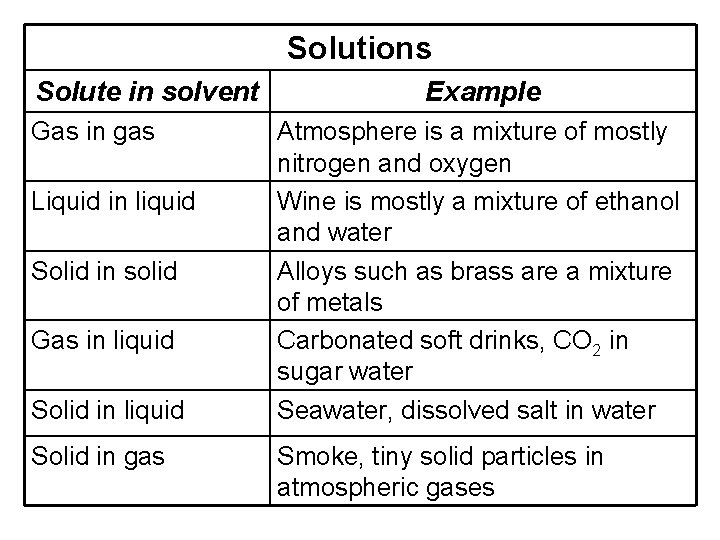
Solutions Solute in solvent Gas in gas Liquid in liquid Solid in solid Gas in liquid Solid in gas Example Atmosphere is a mixture of mostly nitrogen and oxygen Wine is mostly a mixture of ethanol and water Alloys such as brass are a mixture of metals Carbonated soft drinks, CO 2 in sugar water Seawater, dissolved salt in water Smoke, tiny solid particles in atmospheric gases

Steps in Solution Formation 1) Solute particles become separated from other solute particles (the solid). (Energy is absorbed). This is dissociation. 2) Solvent particles become separated from other solvent particles (moved apart to allow solute particles to enter the liquid). (Energy is absorbed) 3) Solvent particles are attracted to and surround solute particles (energy released)— the solute particles are said to be solvated.
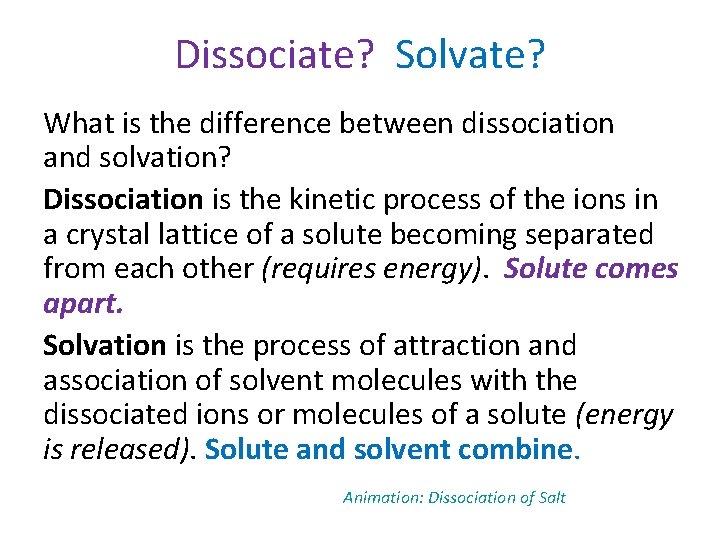
Dissociate? Solvate? What is the difference between dissociation and solvation? Dissociation is the kinetic process of the ions in a crystal lattice of a solute becoming separated from each other (requires energy). Solute comes apart. Solvation is the process of attraction and association of solvent molecules with the dissociated ions or molecules of a solute (energy is released). Solute and solvent combine. Animation: Dissociation of Salt

Factors affecting rate of dissolution If you have ever tried to dissolve sugar in iced tea, you know that there are factors that affect how quickly you can dissolve sugar in iced tea. List 3: 1) Agitating (stirring or shaking) the solution Brings fresh solvent in contact with solute surface 2) Surface area of solute – the more SA, the faster dissolving occurs Powders dissolve more readily than do large crystals 3) Heating the solvent Increases the KE of solvent particles which means more frequent collisions with solute particles to help disperse the solute
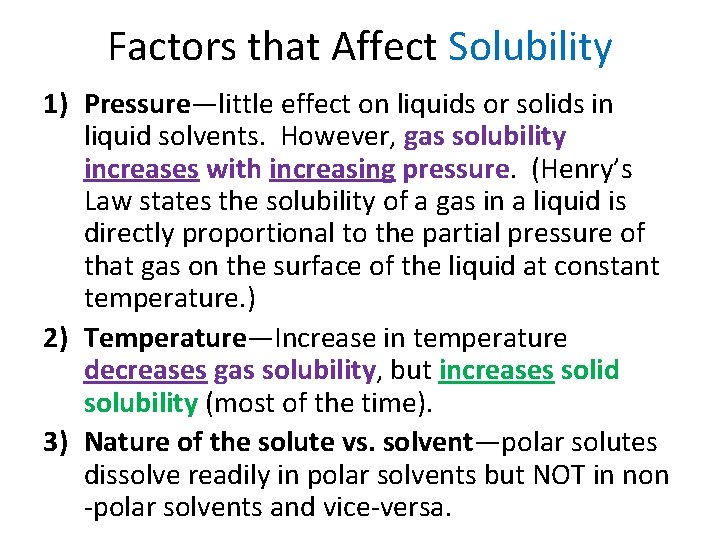
Factors that Affect Solubility 1) Pressure—little effect on liquids or solids in liquid solvents. However, gas solubility increases with increasing pressure. (Henry’s Law states the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas on the surface of the liquid at constant temperature. ) 2) Temperature—Increase in temperature decreases gas solubility, but increases solid solubility (most of the time). 3) Nature of the solute vs. solvent—polar solutes dissolve readily in polar solvents but NOT in non -polar solvents and vice-versa.
Polar vs. Nonpolar Interactions “Like dissolves like” is a useful, but rough, rule for predicting whether one substance will dissolve in another. It is based upon: • Polar vs. nonpolar interactions—polar substances (have a dipole moment or positive and negative ‘ends’) and ionic compounds are generally NOT soluble in nonpolar solvents such as paint remover, kerosene, turpentine, mineral oil, gasoline, and nail polish remover. Instead, polar substances and ionic compounds are soluble in water which is why water is called the ‘universal solvent. ’ • Types of bonding • Intermolecular forces between solute and solvent—ions and polar molecules are linked by dipole-dipole attractions while nonpolar molecules are linked to nonpolar solvents with London dispersion forces.

Electrolytes • A substance that dissolves in water to give a solution that conducts electric current is called an electrolyte. Sodium chloride (Na. Cl) and any other soluble ionic compound is an electrolyte because it contains mobile charged particles. • A substance that dissolves in water to give a solution that does NOT conduct an electric current is called a nonelectrolyte. Sucrose is an example because its dissolved molecules, although mobile, are not ions and do not carry an electric charge.

Strong vs. Weak Electrolytes • Strong electrolytes completely or nearly completely dissociate (form ions) and are EXCELLENT conductors of electricity (e. g. , strong acids and strong bases) • Weak electrolytes only partially dissociate and are POOR conductors of electricity (e. g. , weak acids and weak bases) • Non-electrolytes DO NOT dissociate or form ions and therefore DO NOT conduct electricity (e. g. , sugars, alcohols)

Solution Equilibrium • If you add spoonful after spoonful of sugar to tea, eventually there comes a point where no more sugar will dissolve. For every combination of solute and solvent, there is a limit to the amount of solute that can dissolve. • Why? Eventually the rate at which sugar molecules are leaving the solid surface of the crystal and being carried away by the water is exactly balanced by the rate that dissolved molecules of sugar are returning to and recrystallizing at the crystals of sugar. This dynamic equilibrium is called solution equilibrium and it is the physical state in which the opposing processes of dissolution and crystallization of a solute occur at equal rates.

Lab Techniques to Separate Mixtures • Some mixtures are easier to separate than others. How would you separate tossed salad? Trail mix? Beef stew? Vinaigrette salad dressing? Orange juice? Muddy water? Milk? • Consider the following separation techniques:
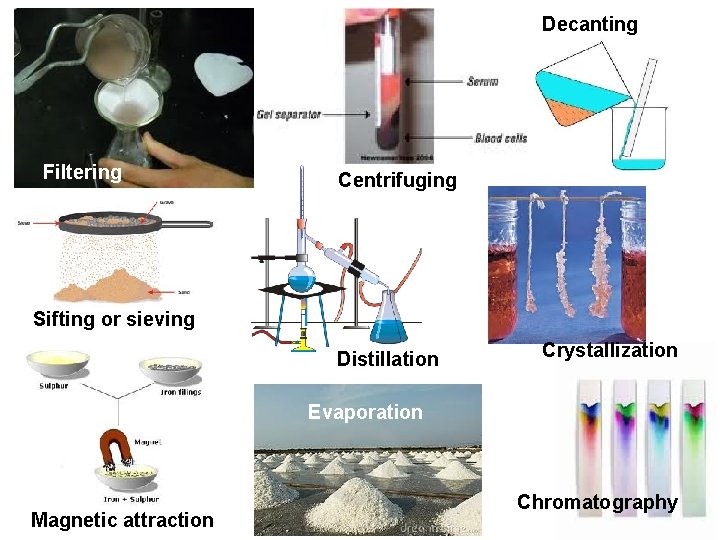
Decanting Filtering Centrifuging Sifting or sieving Distillation Crystallization Evaporation Magnetic attraction Chromatography
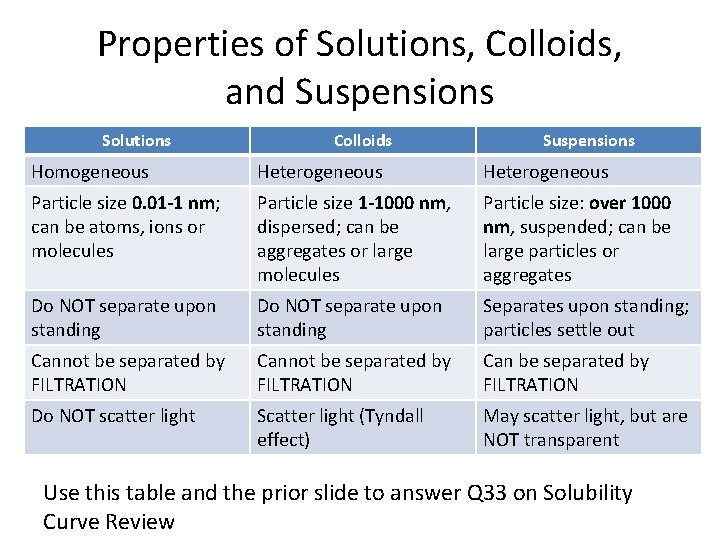
Properties of Solutions, Colloids, and Suspensions Solutions Colloids Suspensions Homogeneous Heterogeneous Particle size 0. 01 -1 nm; can be atoms, ions or molecules Particle size 1 -1000 nm, dispersed; can be aggregates or large molecules Particle size: over 1000 nm, suspended; can be large particles or aggregates Do NOT separate upon standing Separates upon standing; particles settle out Cannot be separated by FILTRATION Can be separated by FILTRATION Do NOT scatter light Scatter light (Tyndall effect) May scatter light, but are NOT transparent Use this table and the prior slide to answer Q 33 on Solubility Curve Review

Dilution • The concentration of a solution is a measure of the amount of solute in a given amount of solvent or solution. • ‘Dilute’ and ‘concentrated’ are words often used without definite meanings. ‘Dilute’ just means there is a relatively small amount of solute compared to the amount of solvent, while ‘concentrated’ means a relatively large amount of solute compared to the amount of solvent.
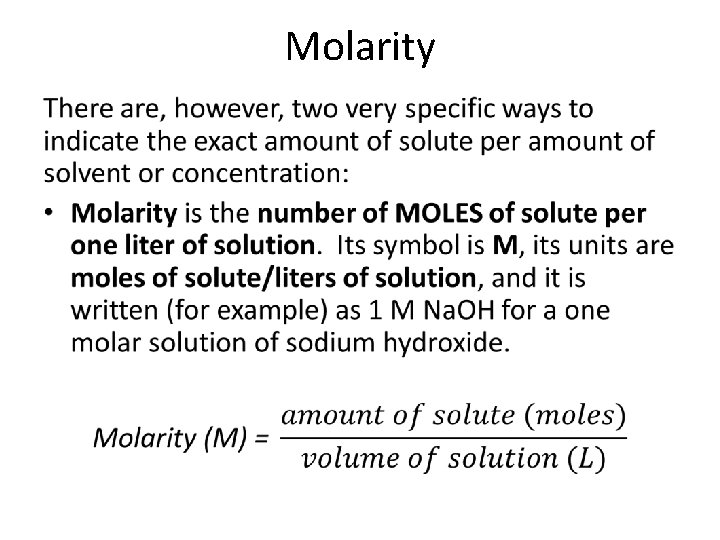
Molarity •

Beware! • Note that a 1 M solution is NOT made by adding 1 mol of solute to 1 L of solvent because the resulting total volume would be MORE THAN 1 L! • Instead, the 1 mol of solute is dissolved in less than 1 L of solvent, then enough solvent is added to the result to bring the total volume to one liter (1 L).
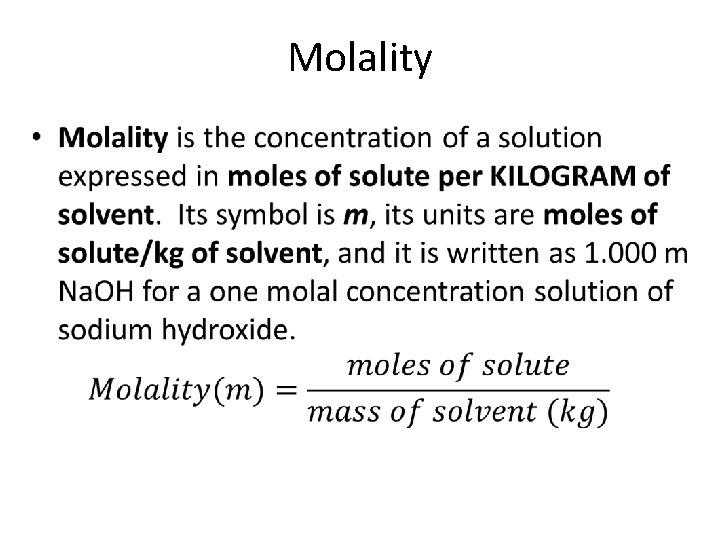
Molality •

Why have both molarity and molality? Concentrations expressed in molalities are used when studying properties of solutions related to vapor pressure and temperature changes because molality is independent of temperature. NOTE: Water is the most common solvent; if the solvent is not specified, assume water. Remember that 1 ml of water has a mass of 1. 000 g, so that 1 L of water has a mass of 1. 000 kg.
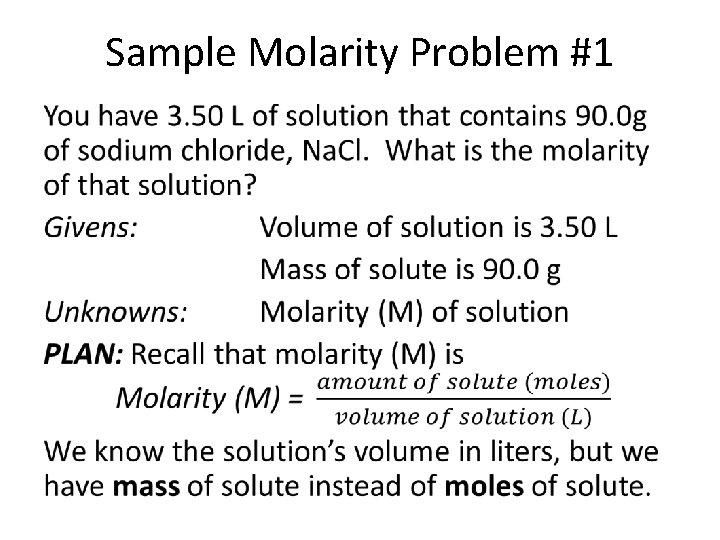
Sample Molarity Problem #1 •
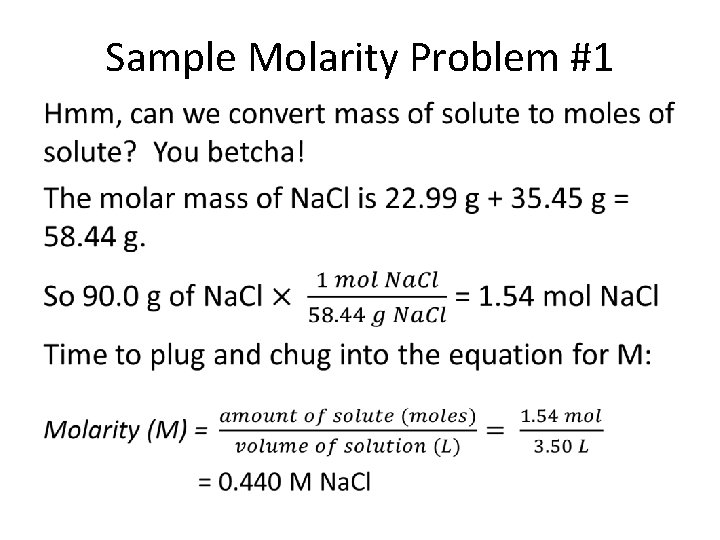
Sample Molarity Problem #1 •

Sample Molarity Problem #2 How many grams of Na. Cl should be used to prepare 300 m. L of a 0. 20 M solution? Givens: volume of solution is 300 m. L (= 0. 3 L) molarity (M) is 0. 20 M Unknown: grams of Na. Cl? PLAN: Use molarity equation to (1) solve for number of moles of solute, then (2) convert moles of solute to grams of solute.
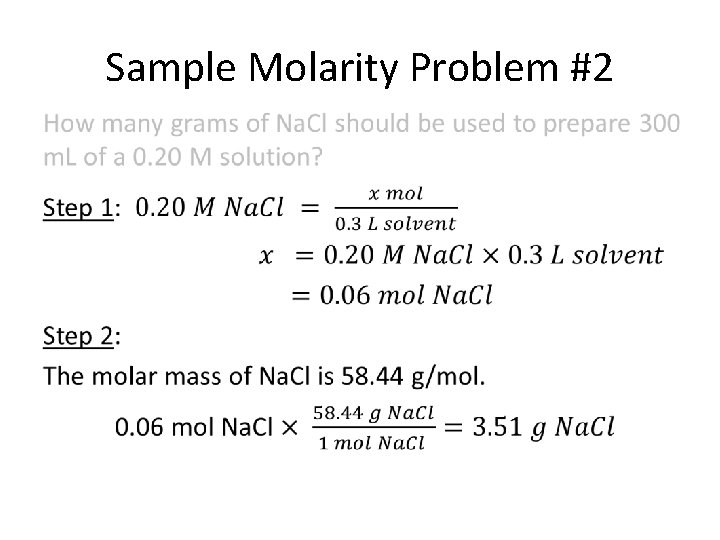
Sample Molarity Problem #2 •

Sample Molality Problem #1 •
Sample Molality Problem #2 How many grams of sucrose (C 12 H 22 O 11) should be added to 125 g of water to prepare a 0. 400 m C 12 H 22 O 11 solution? Givens: 125 g water = mass of solvent 0. 400 m = molality of solution Unknown: mass of solute, sucrose, needed PLAN: Use formula for molality and the molar mass of sucrose (C 12 H 22 O 11) to find the mass of sucrose in a 1. 0 m C 12 H 22 O 11 solution. Then convert to a 0. 400 m solution and finally use a proportion to find mass of solute in 125 g of solvent (first convert g of solvent to kg of solvent).
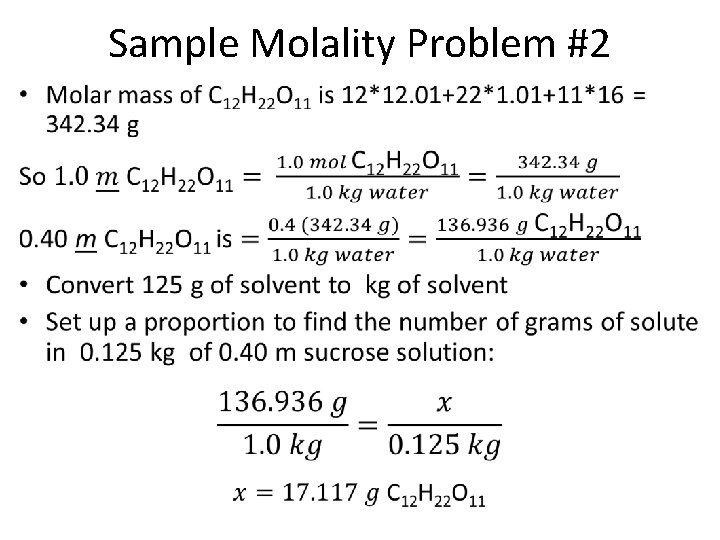
Sample Molality Problem #2 •

Dilution Problems •

Dilution Example •
- Slides: 33