Solubility Constant Solubility Some ionic compounds dissolve in

Solubility Constant

Solubility � Some ionic compounds dissolve in water while other will not � It is not always all or nothing � Others are slightly soluble ◦ A fraction of the ionic compound will dissolve in water while some ◦ A fraction of the compound will dissociate into ions

Equilibrium � The solution of a slightly soluble solid exist in equilibrium � Ions are separating and reforming at constant rate ◦ Every time a molecule dissolves a new one forms from ions reforming � The expression can be written as ◦ AB 2 ⇄ A + 2 B � The reaction goes forward and backward at the same rate

Solubility Constant � At equilibrium, the concentrations of the free ions are known � Mathematical relationship exists between the free ions � For slightly soluble solids the relationship exits ◦ AB 2 A+ + 2 B◦ Ksp = [A+] x [B-]2 � Note the coefficient becomes the exponent � The value for Ksp is known for most subtances
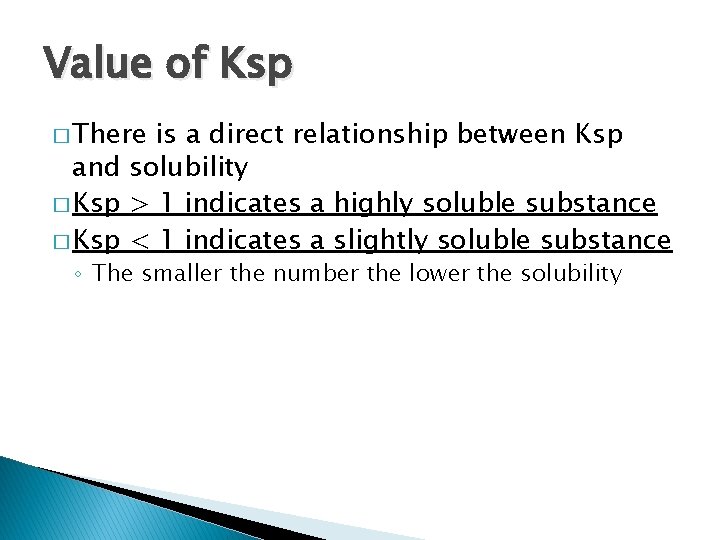
Value of Ksp � There is a direct relationship between Ksp and solubility � Ksp > 1 indicates a highly soluble substance � Ksp < 1 indicates a slightly soluble substance ◦ The smaller the number the lower the solubility
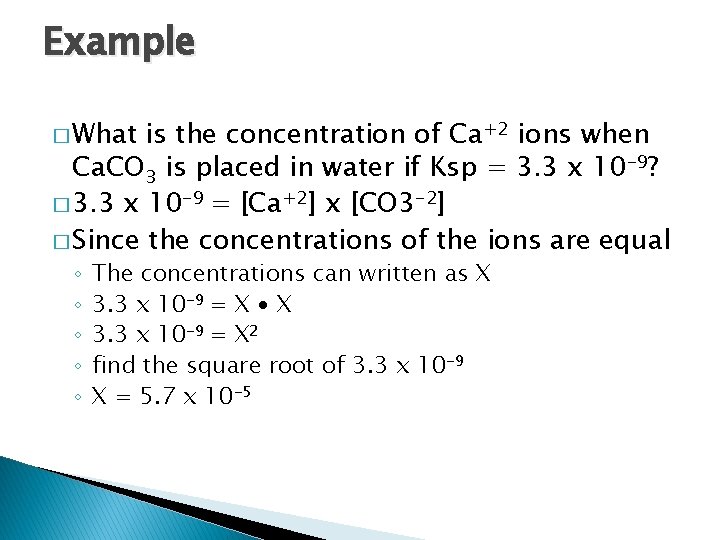
Example � What is the concentration of Ca+2 ions when Ca. CO 3 is placed in water if Ksp = 3. 3 x 10 -9? � 3. 3 x 10 -9 = [Ca+2] x [CO 3 -2] � Since the concentrations of the ions are equal ◦ ◦ ◦ The concentrations can written as X 3. 3 x 10 -9 = X ∙ X 3. 3 x 10 -9 = X 2 find the square root of 3. 3 x 10 -9 X = 5. 7 x 10 -5
- Slides: 6