Solubility Conductivity and Polarity What is Solution Chemistry

Solubility, Conductivity, and Polarity

What is Solution Chemistry? • A solution is a homogenous mixture – A homogeneous mixture is one where particles are evenly mixed and you cannot use your vision to tell it apart • There are two components in a solution – Solute – Smaller quantity in solution – Solvent – Larger quantity in solution • If the solvent is water, we call it an aqueous solution

What is Solution Chemistry? • In general, we typically dissolve a solid (solute) in a liquid (solvent) which is usually water. • We use the word soluble to mean when the solute can dissolve in the solvent to create a homogeneous mixture – It is insoluble when it cannot dissolve or only a little is dissolved and a lot of solute is left not dissolved
What is Solution Chemistry? • The nature of solutions allows us to modify the concentration to suit our needs in an experiment • The amount of one substance that will dissolve in a certain amount of another at a specific temperature refers to the solubility of the substance – This is measured in grams per litre or grams per 100 m. L • Solubility varies depending on temperature – This is due to kinetic molecular theory as particles move faster, so reactions increase in speed

What is Solution Chemistry? • Now what type of solids dissolve into solutions? • Typically they are ionic salts • When ionic salts dissolve into a solution, they dissociate into their charged particles. – Metals become cations – Non-metals becomes anions • They are no longer in their crystal lattice

Saturated and Unsaturated • When no more solute can dissolve in the solvent, we say the solution is saturated • When more solute can dissolve, we say the solution is unsaturated • We typically have some undissolved solute present in a saturated solution due to temperature changes. If the temperature increases, it can still remain saturated

Recall - Dilutions • Remember that we can dilute chemicals or mix two chemicals together • C 1 V 1 = C 2 V 2 • We can also write it as • M 1 V 1 = M 2 V 2 • Or even Ci. Vi = Cf. Vf

Example - 1 • A student has 500. 0 m. L of a 0. 40 M Na. Cl solution. How much water is needed to make it a 0. 10 M solution?

Example - 2 • A chemist adds water to 120. 0 m. L of a 6. 00 M solution of Na. OH until the final volume is 2. 00 L. What is the molarity of the resulting solution?

Example - 3 • What concentration results when 150. 0 m. L of 0. 36 M solution of Mg. SO 4 are added to 750. 0 m. L of water?

Example - 4 • A chemist mixes 600. 0 m. L of 0. 500 M of Na. OH with 900. 0 m. L of 0. 750 M of Na. OH. What was the resulting concentration?

Solubility • Solubility is the maximum amount of a solute which can dissolve in a given amount of solvent at a given temperature • We typical measure this at g/100 m. L or g/L • Solubility changes based on what you dissolve it in • Na. Cl has different solubility based on what it is dissolved in – Water = 35. 9 g/100 m. L – Ammonia = 2. 15 g/100 m. L – Methanol = 1. 49 g/100 m. L
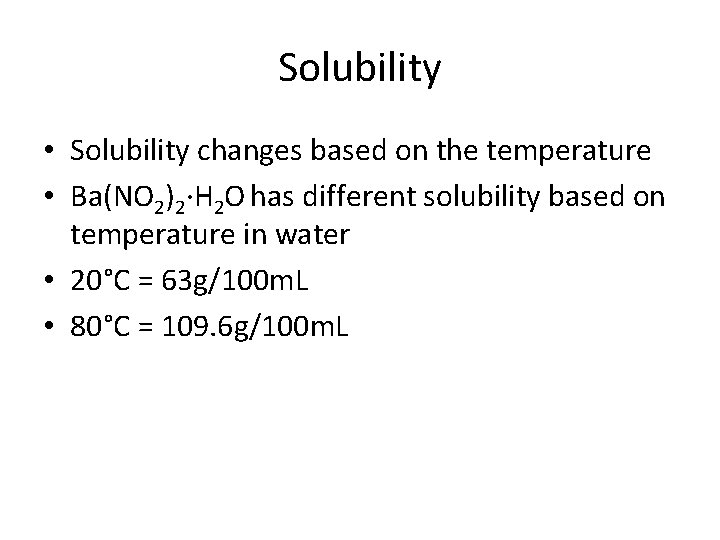
Solubility • Solubility changes based on the temperature • Ba(NO 2)2∙H 2 O has different solubility based on temperature in water • 20°C = 63 g/100 m. L • 80°C = 109. 6 g/100 m. L
Defining Solubility • • • The type of solute used The amount of solute The type of solvent used The amount of solvent What the temperature of the solution is

Practice - 1 • Page 194 - #1 -4

Conductivity • When the ionic salts dissolve in solution, they can conduct electricity – Ions are charged particles – They are charged due to the transfer of electrons! – Electricity is the flow of electrons • The greater the concentration of ions, the greater the conductivity • When the solute is not an ionic salt, it will not conduct electricity.

Conductivity • Ionic Salts – Na. Cl – Mg. Cl 2 – Mg. O • Compounds that are not ionic solids and contain carbon typically do not conduct – Few exceptions being CH 3 COOH (acetic acid/vinegar) – Acids and bases conduct as they dissociate into ions too
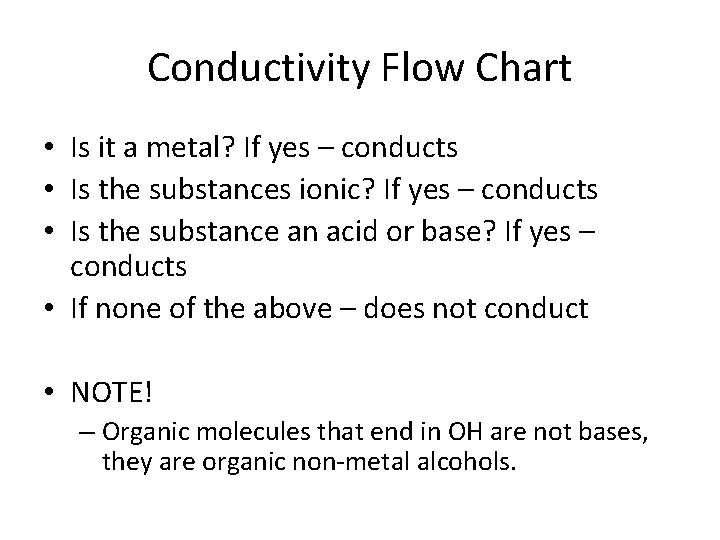
Conductivity Flow Chart • Is it a metal? If yes – conducts • Is the substances ionic? If yes – conducts • Is the substance an acid or base? If yes – conducts • If none of the above – does not conduct • NOTE! – Organic molecules that end in OH are not bases, they are organic non-metal alcohols.

Practice - 2 • Page 198 - #6 -8
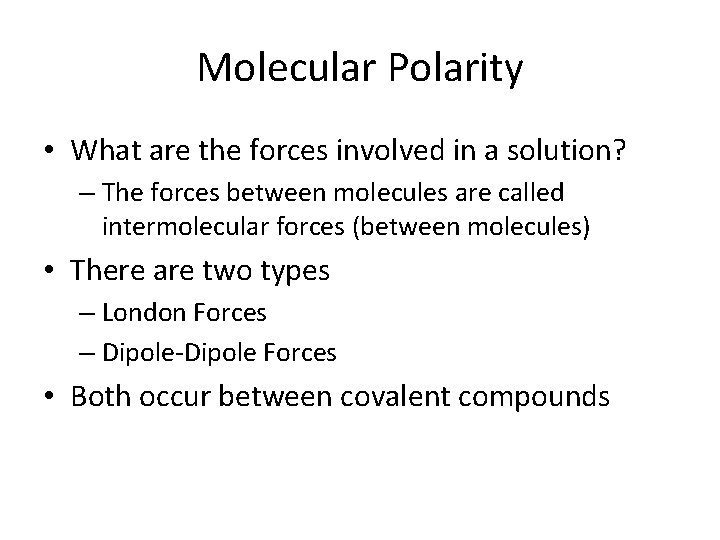
Molecular Polarity • What are the forces involved in a solution? – The forces between molecules are called intermolecular forces (between molecules) • There are two types – London Forces – Dipole-Dipole Forces • Both occur between covalent compounds

London Forces • Uses the idea of temporary dipoles – In covalent compounds, there is a spread of electrons around the atom. – This creates some temporary attraction as the electrons move around • Remember electrostatic forces – Opposite charges attract • With these temporary positive and negative ends, they will attract and bond – These are called London Forces

Dipole-Dipole Forces • Dipole-dipole forces are result of molecules have permanent dipoles – Covalent compounds • Molecules with dipoles have atoms with differences in electronegativity between the two atoms – Due to this, you will have one end of the molecule being more negative and the other end being more positive • If there are no permanent dipole, then only London Forces are present
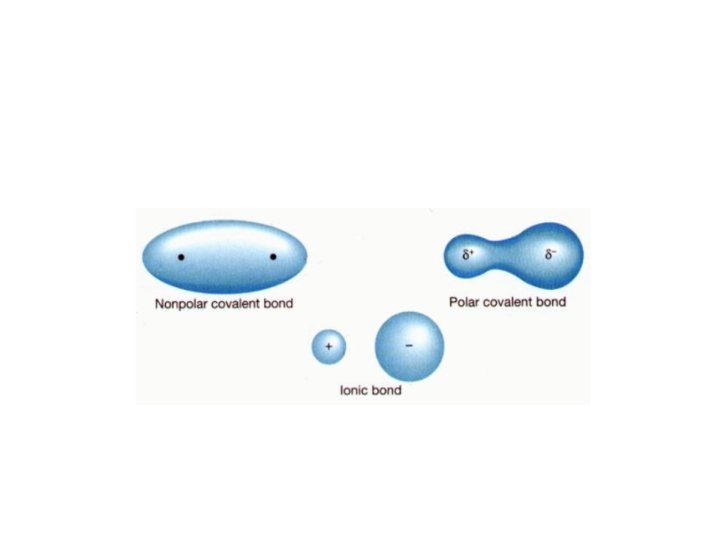
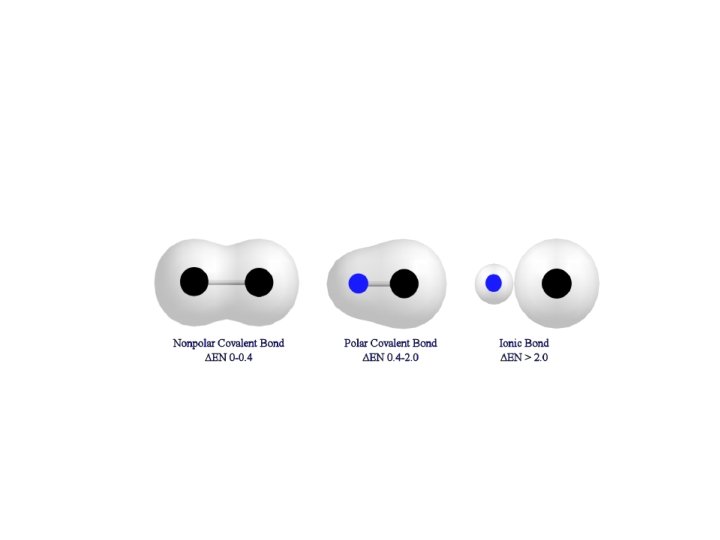
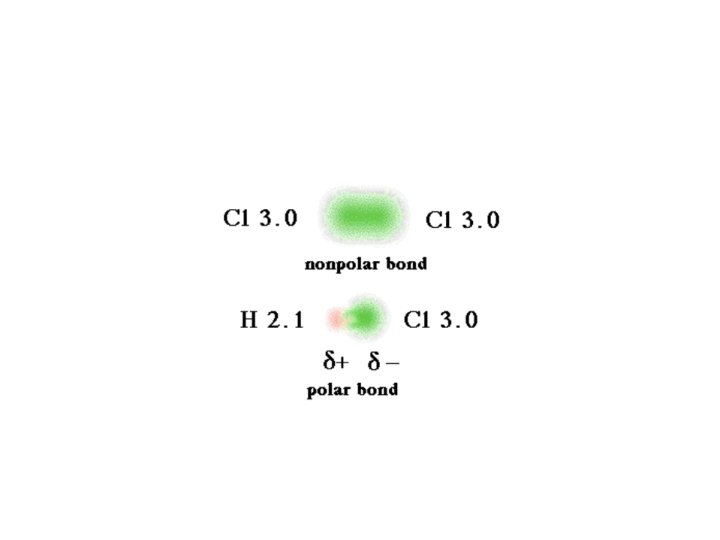

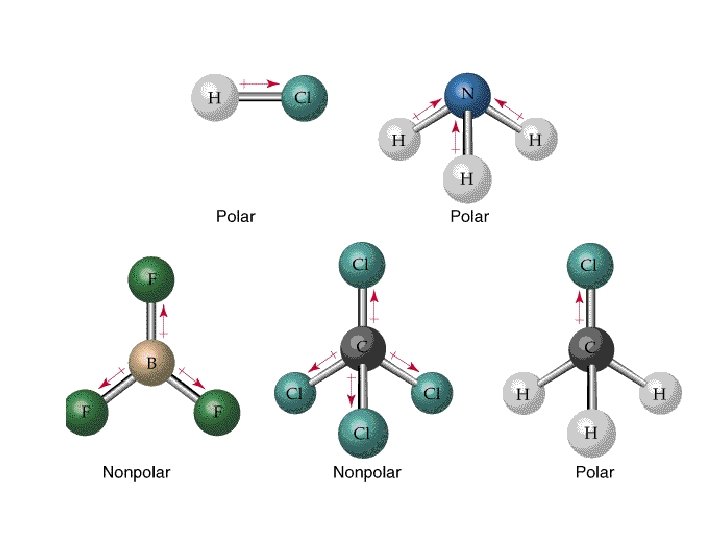
Dipole-Dipole Forces • A molecule that has a partial negative and positive end is said to be polar • A molecule that is not polar, that means does not have a negative or positive end is called non-polar

Practice - 3 • Page 199 - #9
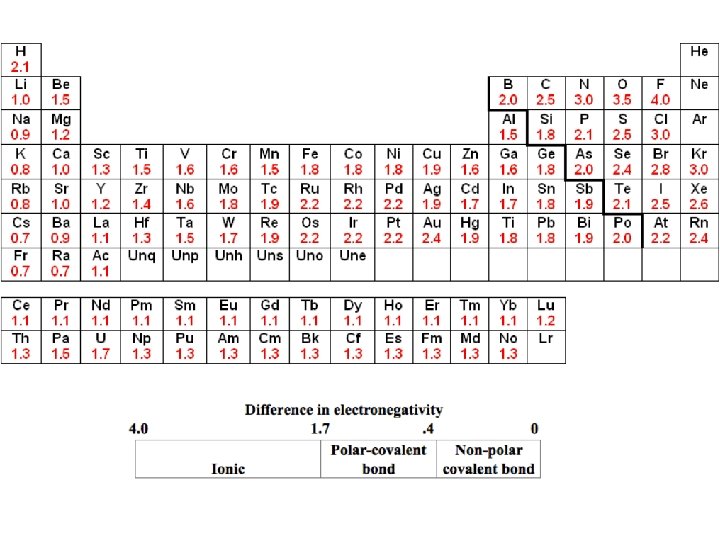
Electronegativity and Polarity • For a molecular to be polar, there must be a difference in electronegativity and the molecule must be asymmetrical • If the molecule is symmetrical, it is non-polar – Think of tug-o-war

Practice - 4 • Page 201 - #10

Dipole-Dipole vs Ionic • Due to ionic being a difference of charge, they are much stronger than dipole-dipole forces • A +1 and a -1 charge will be much more attracted than a difference in electronegativity • This is why ionic compounds have a higher melting point • Polar molecules will also have a higher melting point than non-polar molecules

Hydrogen Bonding • It is a type of dipole-dipole force that occurs between the hydrogen atom in a molecule that is attached to N, O, or F and another N, O, F from another molecule • It is present in molecules that contain H-N, H-O, or H-F bonds. • An example is water and holding the double helix of DNA together • Hydrogen bonds are weaker than covalent bonds or ionic bonds, but stronger than regular dipole

Sample - 1 • This is how hydrogen bonds work with water H 2 O

Practice - 5 • Page 203 - #13 -16

Polar and Non-polar Solvents • Why does like dissolve like? • Ionic solutes (e. g. Na. Cl) are held together by strong bonds • Non-polar solvents have only London Forces between molecules – too weak to break apart the ionic solute bonds and pull them apart • Polar solvents have dipole-dipole forces between them – strong enough to dissolve ionic solids and break their bonds

Polar and Non-polar Solvents • This is true for polar solutes as with solvents • Non-polar solutes are only attracted by London Forces – But they have no dipole. No positive and negative ends – They do not attract to polar solvents – Thus they do not “react” with polar solvents so polar solvents cannot dissolve them and break their bonds • Only non-polar solvents have STRONG London Forces to break apart non-polar solutes

Practice - 6 • Page 208 - #23 -27
- Slides: 38