Solubility Bell Ringer What is KoolAid made up

Solubility

Bell Ringer § What is Kool-Aid made up of?

https: //phet. colorado. edu/sims/html/concentration/latest/concentration_en. html Phet Concentration https: //phet. colorado. edu/en/simulation/legacy/sugar-and-salt-solutions Sugar and Salt Solutions https: //phet. colorado. edu/en/simulation/legacy/soluble-salts Salts and Solubility

How is Kool-Aid made? Pitcher of Water + Kool-Aid Mix Q 1: How would you compare the volume of water vs. the volume of mix? Q 2: What happens to the mix when added to the water? Q 3: Besides making Kool-aid, what else have I made? Q 4: What would happen to the color if I added more Kool -aid mix?
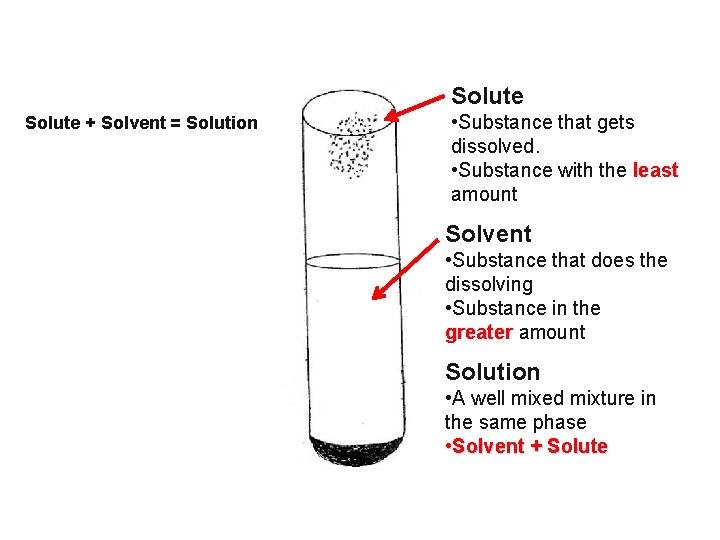
Solute + Solvent = Solution • Substance that gets dissolved. • Substance with the least amount Solvent • Substance that does the dissolving • Substance in the greater amount Solution • A well mixed mixture in the same phase • Solvent + Solute

How is Kool-Aid made? Pitcher of Water + Kool-Aid Mix Q 1: How would you compare the volume of water vs. the volume of mix? Q 2: What happens to the mix when added to the water? Q 3: Besides making Kool-aid, what else have I made? Q 4: What would happen to the color if I added more Kool -aid mix? Q 5: Can I add sugar to the Kool-aid? What will happen to it? Q 6: Is there a limit to the amount of sugar I can add and have it still dissolve?
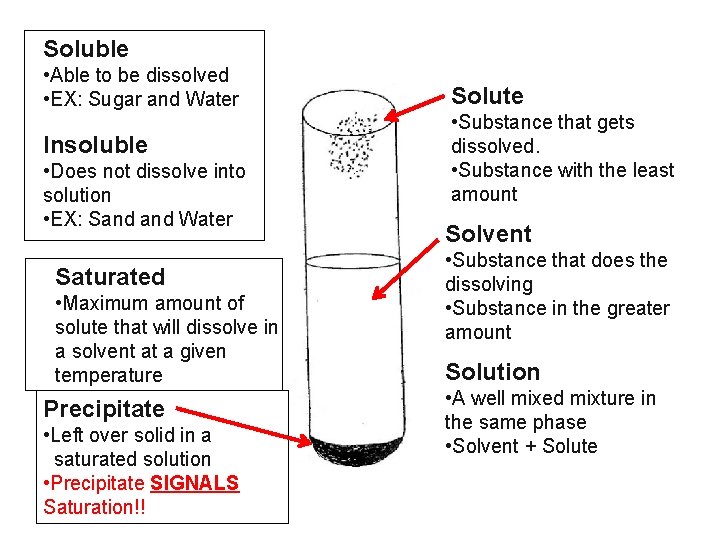
Soluble • Able to be dissolved • EX: Sugar and Water Insoluble • Does not dissolve into solution • EX: Sand Water Saturated • Maximum amount of solute that will dissolve in a solvent at a given temperature Precipitate • Left over solid in a saturated solution • Precipitate SIGNALS Saturation!! Solute • Substance that gets dissolved. • Substance with the least amount Solvent • Substance that does the dissolving • Substance in the greater amount Solution • A well mixed mixture in the same phase • Solvent + Solute

Q 7: How can you make a substance dissolve better? Q 8: When making a glass of chocolate milk, which is the solvent and which is the solute (syrup or milk)? Q 9: How can you make a saturated solution with chocolate milk?

Solubility § The ability of a solute to dissolve § Some substances very soluble in water § Others not very soluble, or insoluble § The process of mixing a solute in a solvent is called dissolving § Insoluble examples: glass or sand in water

Where does salt “go” when it dissolves in water? Water is H 2 O. Salt is Na. Cl, sodium chloride. Positive sodium ions attract to the negative end of a water molecule. Negative chloride ions attract to the positive end of a water molecule. One by one the salt crystals get ‘pulled apart’. They go into the spaces between the water molecules. Disassociation

Mixtures
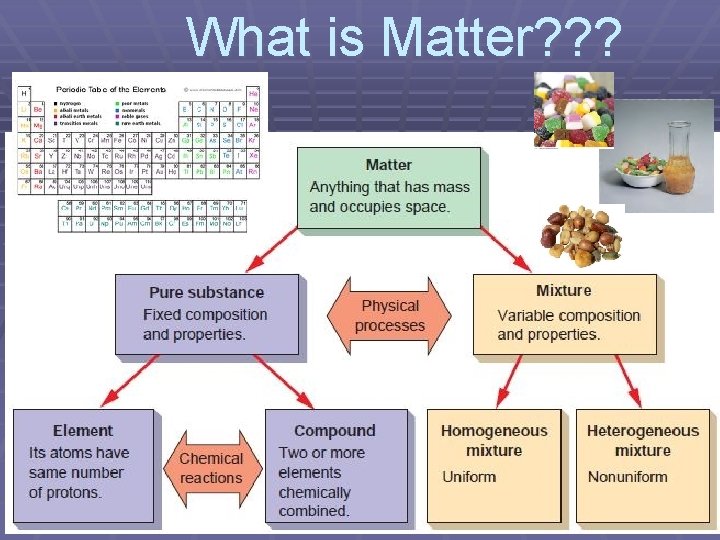
What is Matter? ? ?
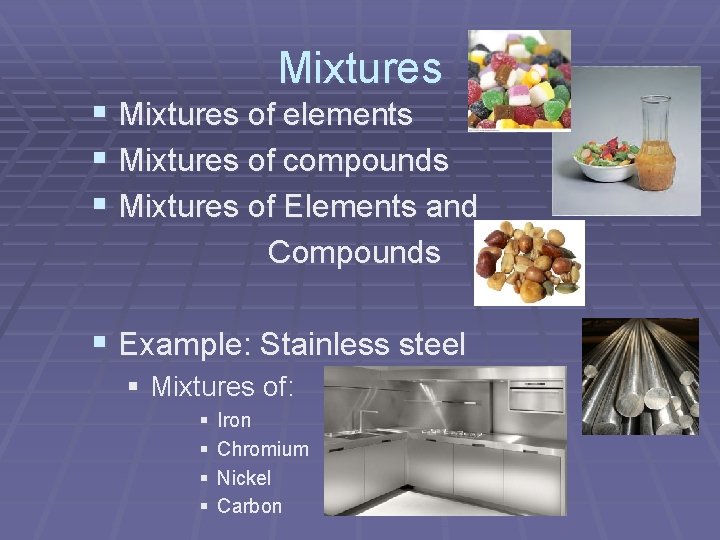
Mixtures § Mixtures of elements § Mixtures of compounds § Mixtures of Elements and Compounds § Example: Stainless steel § Mixtures of: § § Iron Chromium Nickel Carbon

Mixtures Homogeneous Looks like it might be just one substance Heterogeneous Non-uniform Can easily see multiple substances Solution ◦ Air ◦ Salt Water ◦ White Gold Suspension ◦ Milk ◦ Blood ◦ Fog Examples: ◦ Sand in water ◦ Oil and water ◦ Sand salt

Solution § A homogenous mixture in which all components are of the same phase § Homogeneous – evenly mixed § “homo” = “same” § Example: salt water, food coloring & water, air (oxygen, nitrogen, CO 2…)
Solutions & Mixtures

Re-Cap

Solvent § The component in a solution present in the largest amount § Examples: water, nitrogen (largest % of air) Solvent

Solute § Anything in a solution that is not the solvent § Examples: salt, sugar, food coloring

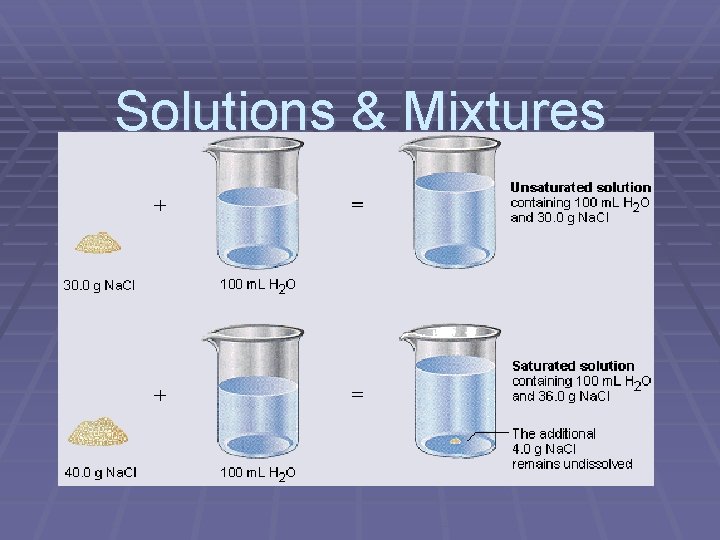
Saturated § A solution containing as much solute as will dissolve § A precipitate signals saturation!!!

Precipitate § A solute that has come out of solution § Above the saturation point, any extra solute is precipitate § Example: rock candy = precipitate
“If you’re not part of the solution, you’re part of the problem. ”
- Slides: 22