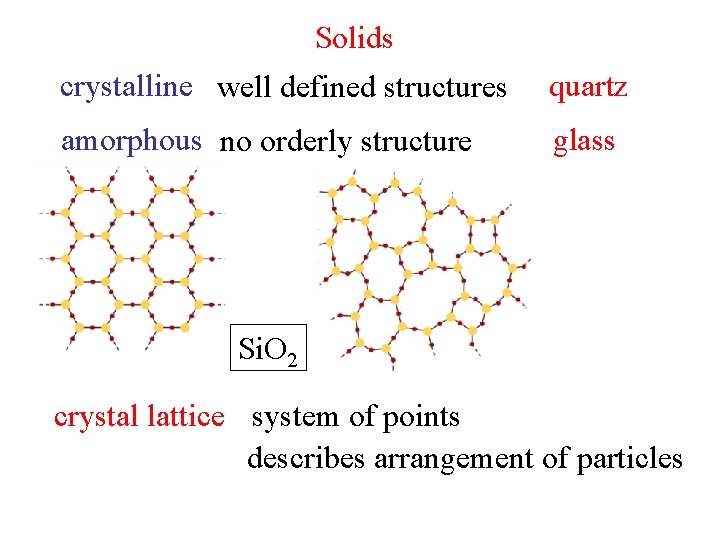
Solids crystalline well defined structures quartz amorphous no
- Slides: 12

Solids crystalline well defined structures quartz amorphous no orderly structure glass Si. O 2 crystal lattice system of points describes arrangement of particles

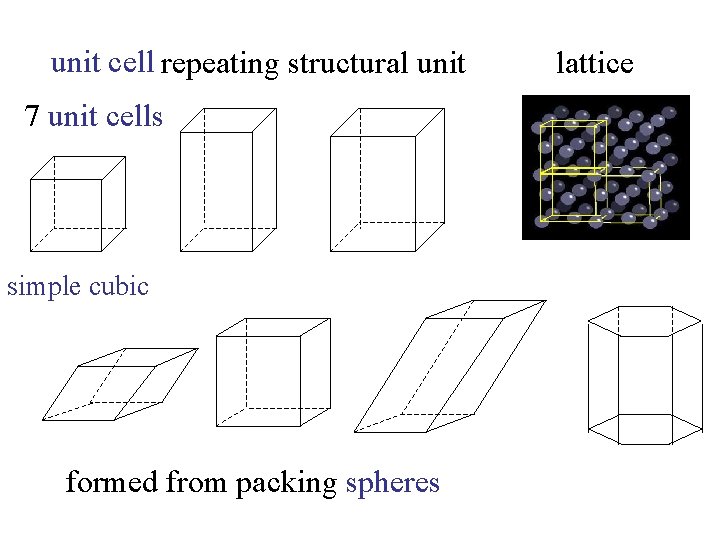
unit cell repeating structural unit 7 unit cells simple cubic formed from packing spheres lattice

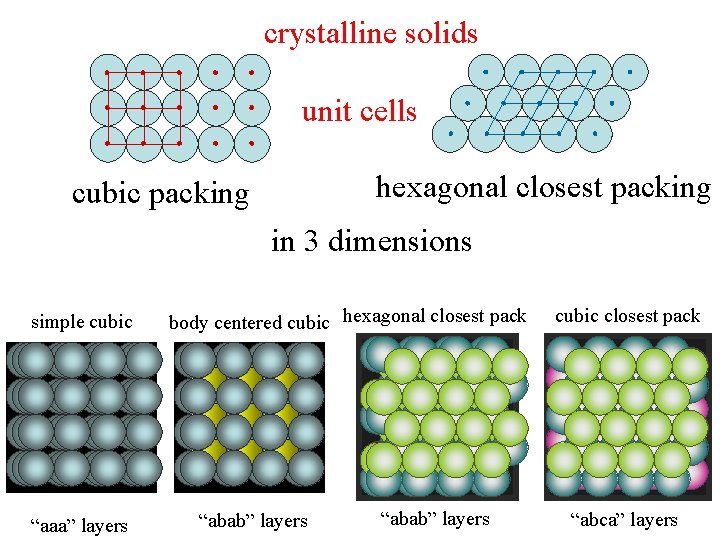
crystalline solids unit cells hexagonal closest packing cubic packing in 3 dimensions simple cubic “aaa” layers body centered cubic hexagonal closest pack “abab” layers cubic closest pack “abca” layers

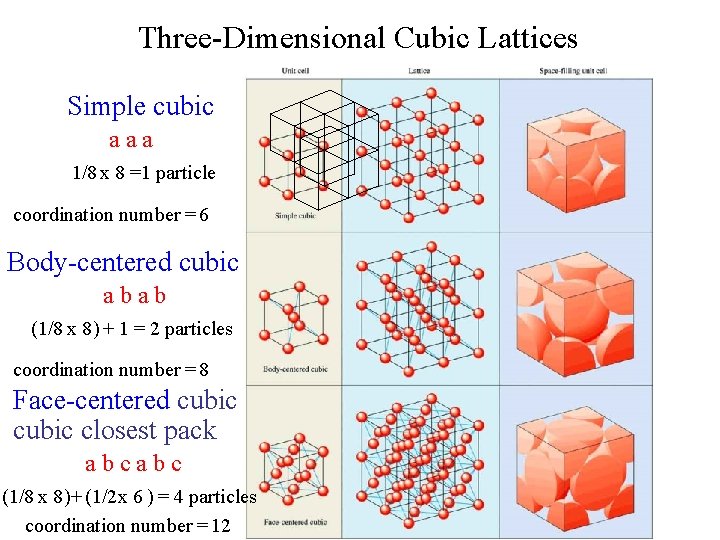
Three-Dimensional Cubic Lattices Simple cubic aaa 1/8 x 8 =1 particle coordination number = 6 Body-centered cubic abab (1/8 x 8 ) + 1 = 2 particles coordination number = 8 Face-centered cubic closest pack abcabc (1/8 x 8 ) + (1/2 x 6 ) = 4 particles coordination number = 12

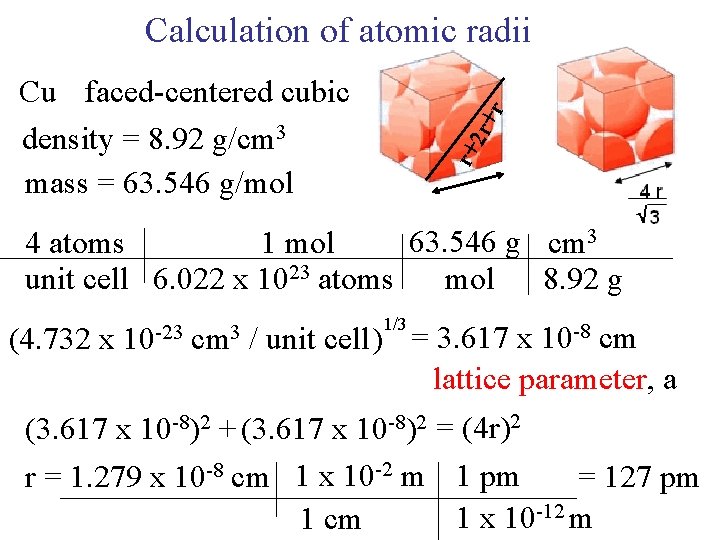
Calculation of atomic radii r+ 2 r+ r Cu faced-centered cubic density = 8. 92 g/cm 3 mass = 63. 546 g/mol 63. 546 g cm 3 4 atoms 1 mol unit cell 6. 022 x 1023 atoms mol 8. 92 g (4. 732 x 10 -23 cm 3 1/3 / unit cell ) = 3. 617 x 10 -8 cm lattice parameter, a (3. 617 x 10 -8)2 + (3. 617 x 10 -8)2 = (4 r)2 r = 1. 279 x 10 -8 cm 1 x 10 -2 m 1 pm = 127 pm 1 x 10 -12 m 1 cm

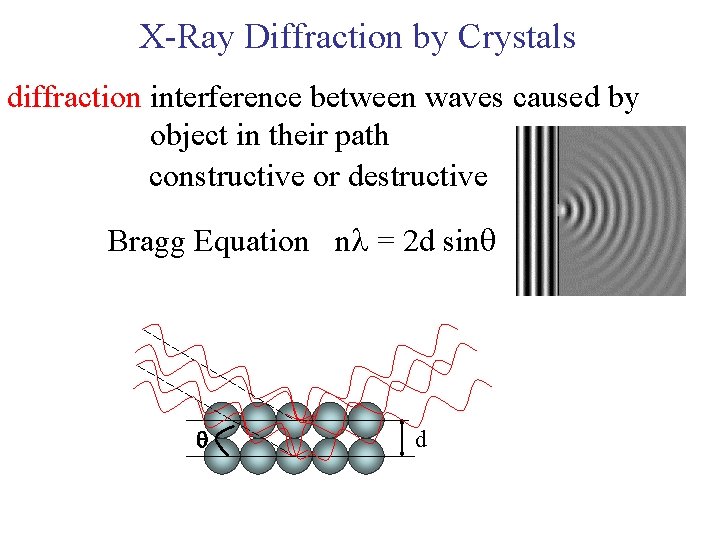
X-Ray Diffraction by Crystals diffraction interference between waves caused by object in their path constructive or destructive Bragg Equation n = 2 d sin d

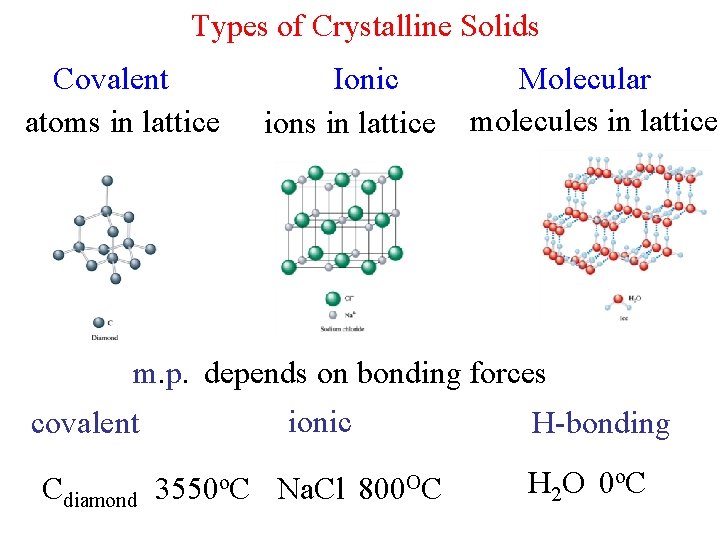
Types of Crystalline Solids Covalent atoms in lattice Ionic ions in lattice Molecular molecules in lattice m. p. depends on bonding forces ionic covalent Cdiamond 3550 o. C Na. Cl H-bonding 800 OC H 2 O 0 o. C

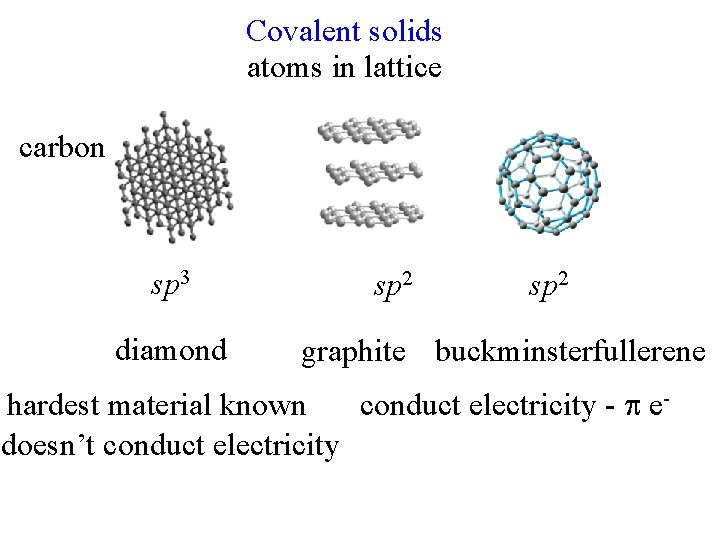
Covalent solids atoms in lattice carbon sp 3 diamond sp 2 graphite buckminsterfullerene hardest material known conduct electricity - edoesn’t conduct electricity

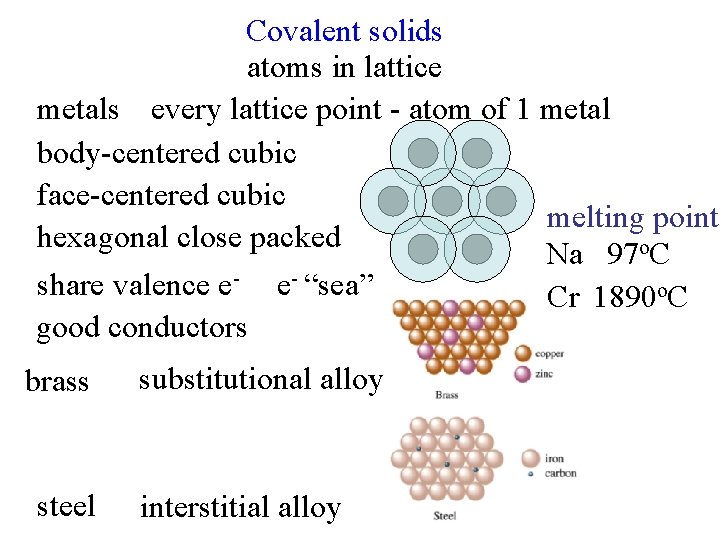
Covalent solids atoms in lattice metals every lattice point - atom of 1 metal body-centered cubic face-centered cubic melting point hexagonal close packed Na 97 o. C share valence e- e- “sea” Cr 1890 o. C good conductors brass steel substitutional alloy interstitial alloy

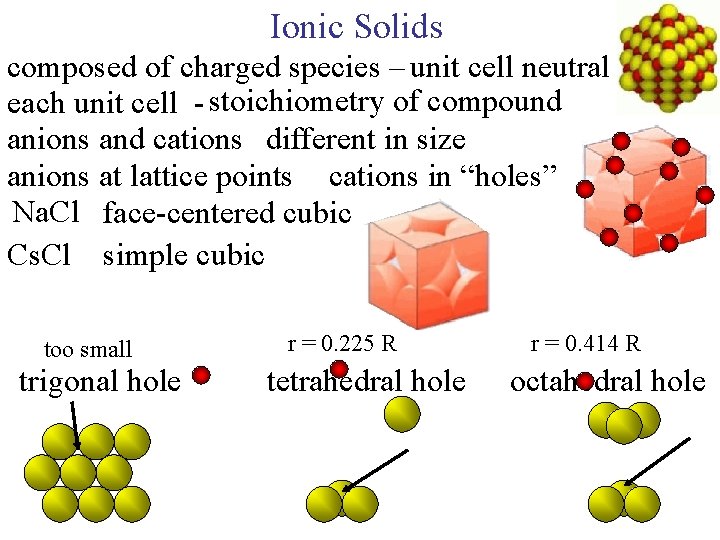
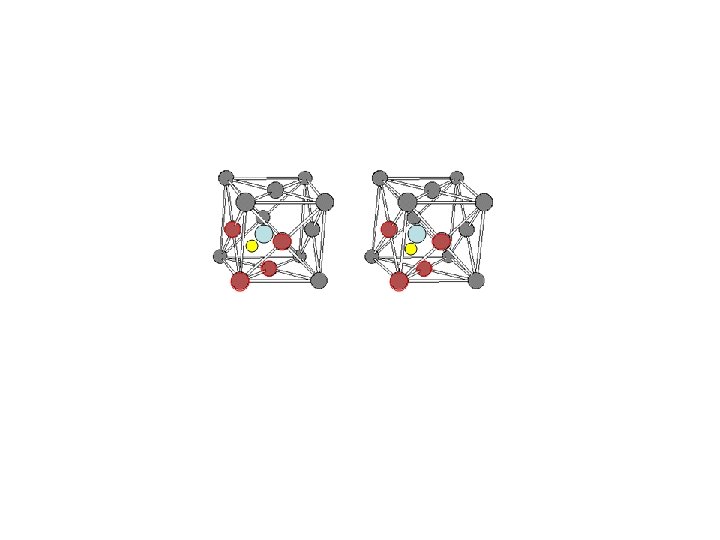
Ionic Solids composed of charged species – unit cell neutral each unit cell - stoichiometry of compound anions and cations different in size anions at lattice points cations in “holes” Na. Cl face-centered cubic Cs. Cl simple cubic too small trigonal hole r = 0. 225 R tetrahedral hole r = 0. 414 R octahedral hole


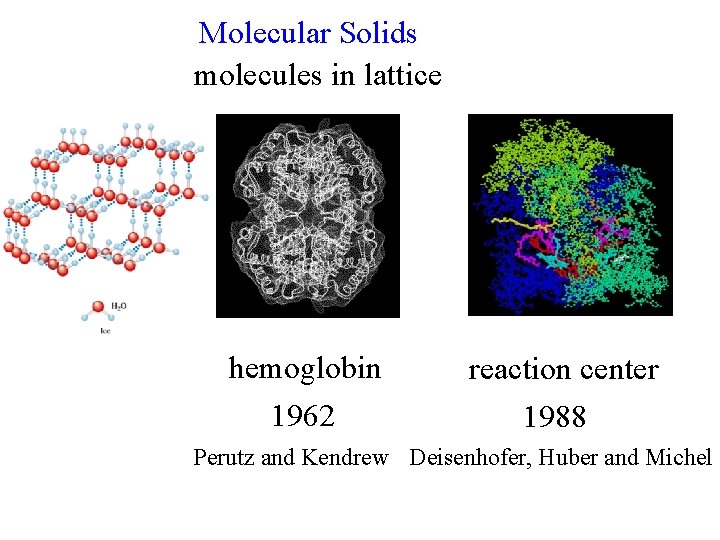
Molecular Solids molecules in lattice hemoglobin 1962 reaction center 1988 Perutz and Kendrew Deisenhofer, Huber and Michel