Solids Crystal Structures the structure of solids crystalline





- Slides: 36

Solids & Crystal Structures

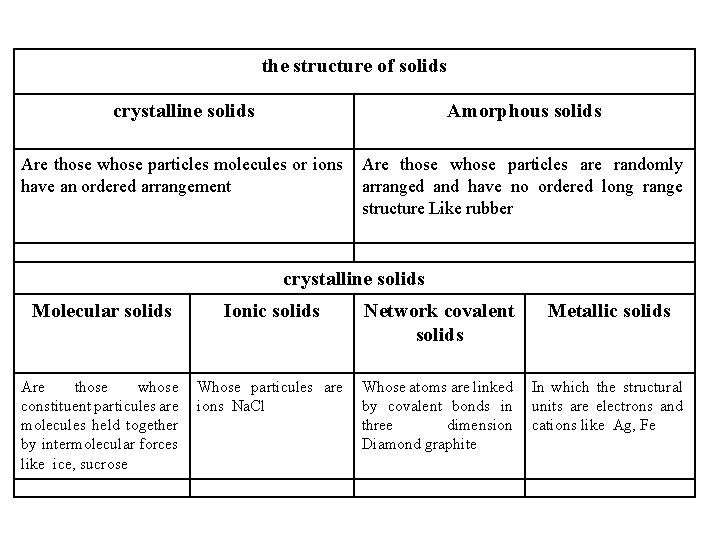
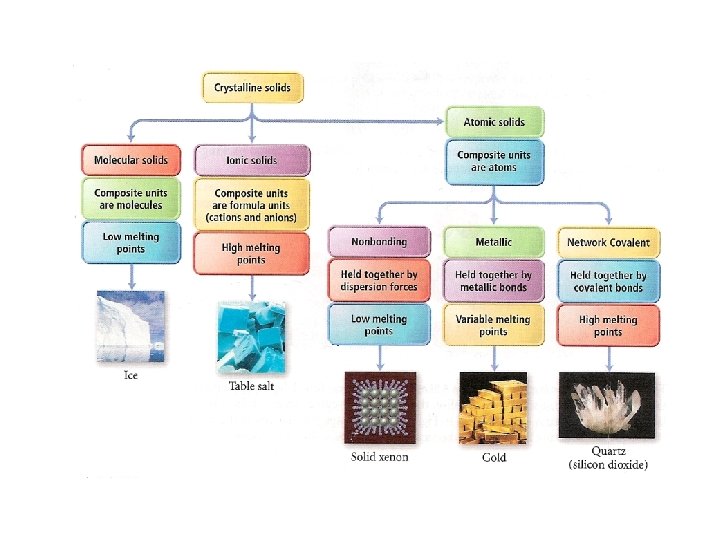
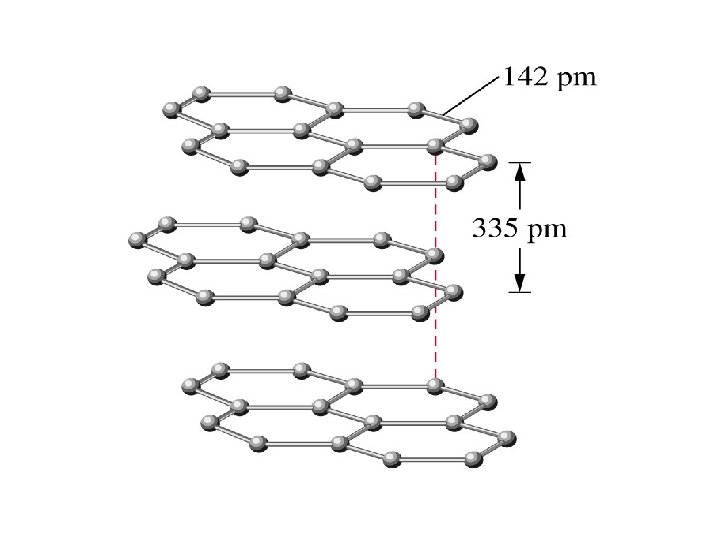
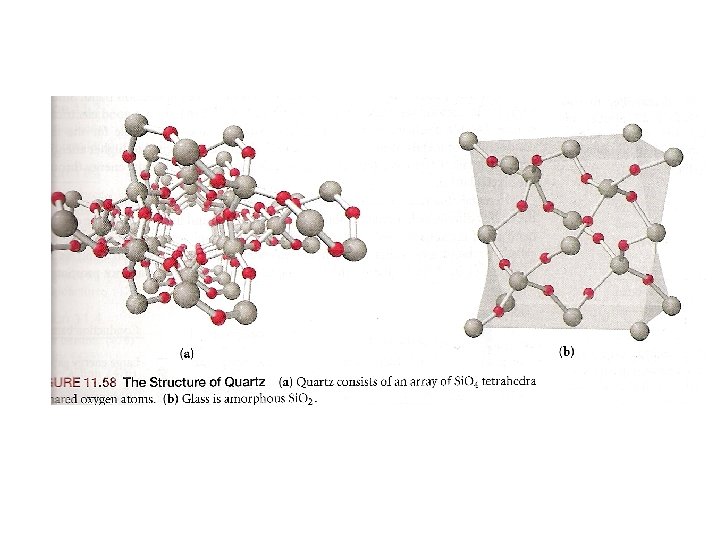
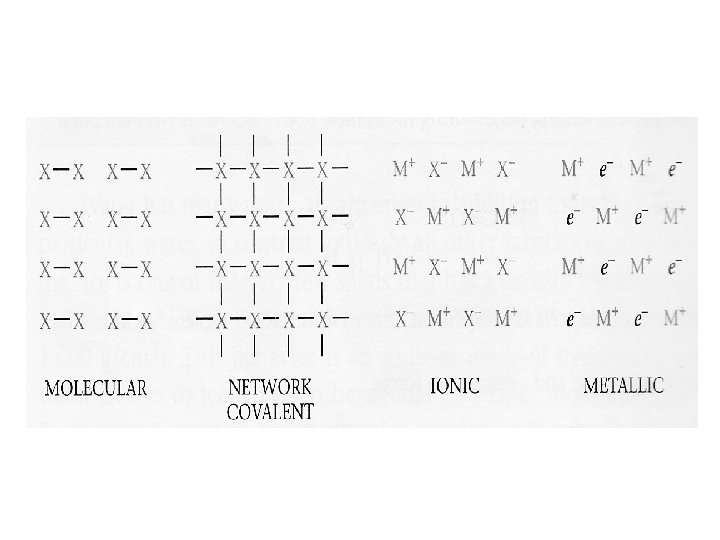
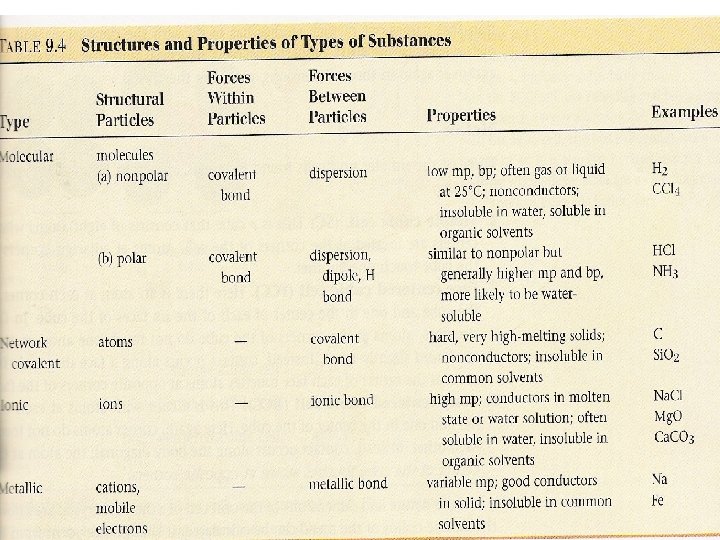
the structure of solids crystalline solids Amorphous solids Are those whose particles molecules or ions have an ordered arrangement Are those whose particles are randomly arranged and have no ordered long range structure Like rubber crystalline solids Molecular solids Ionic solids Network covalent solids Metallic solids Are those whose constituent particules are molecules held together by intermolecular forces like ice, sucrose Whose particules are ions Na. Cl Whose atoms are linked by covalent bonds in three dimension Diamond graphite In which the structural units are electrons and cations like Ag, Fe










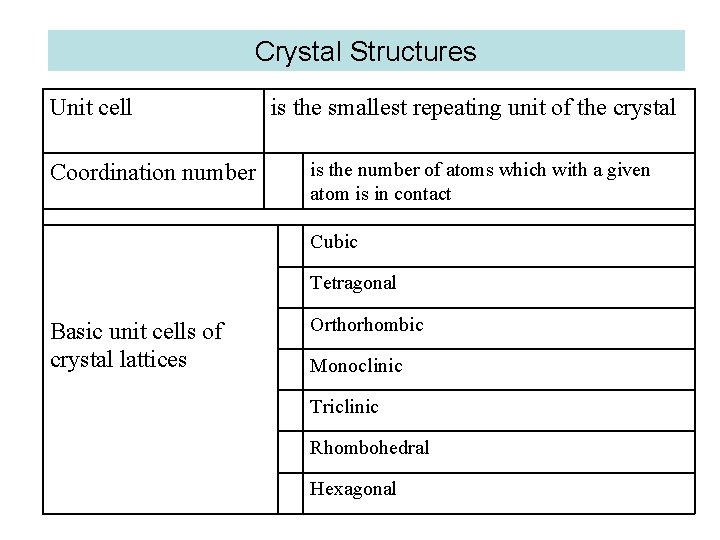
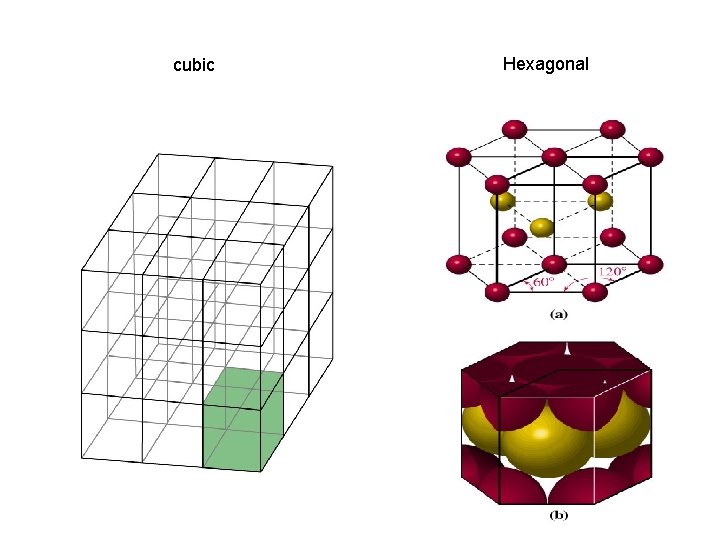
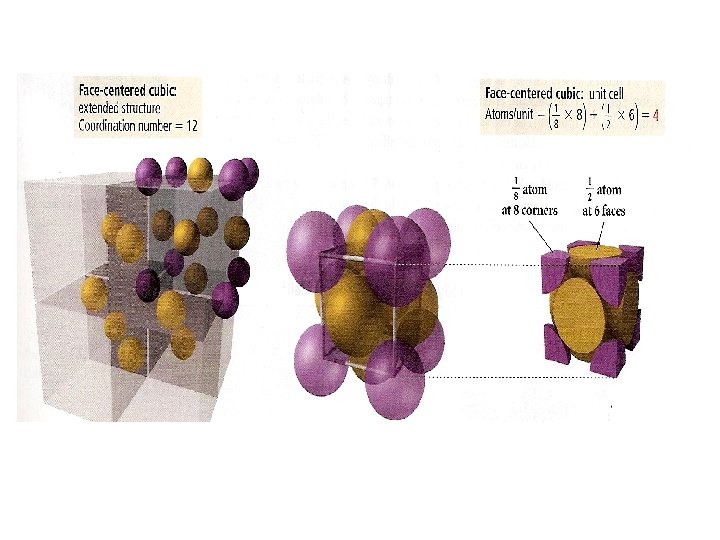
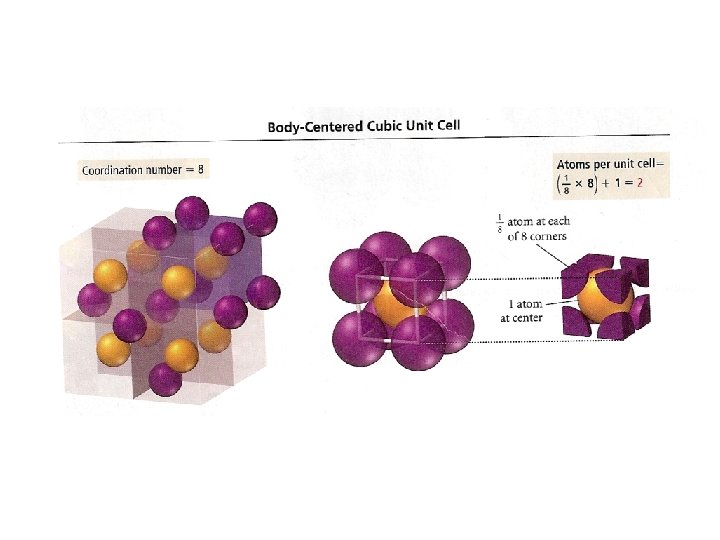
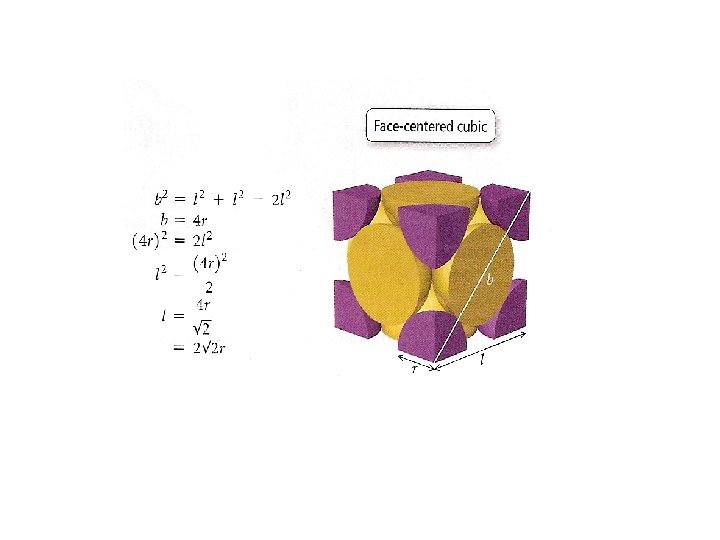
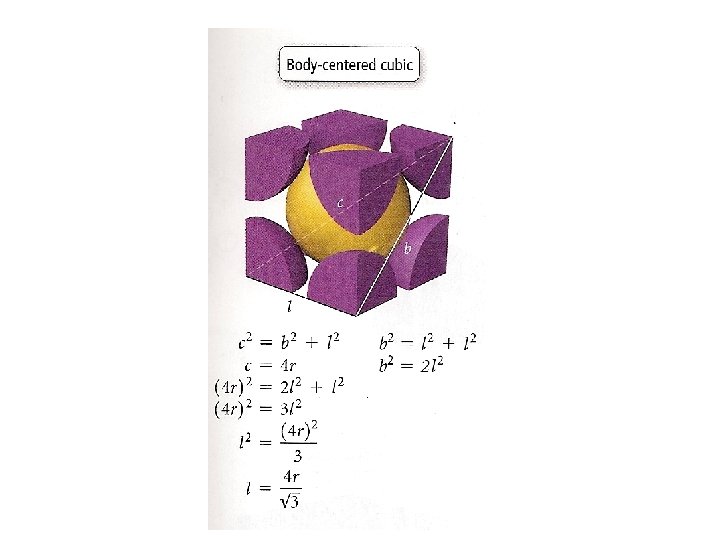
Crystal Structures Unit cell Coordination number is the smallest repeating unit of the crystal is the number of atoms which with a given atom is in contact Cubic Tetragonal Basic unit cells of crystal lattices Orthorhombic Monoclinic Triclinic Rhombohedral Hexagonal

cubic Hexagonal

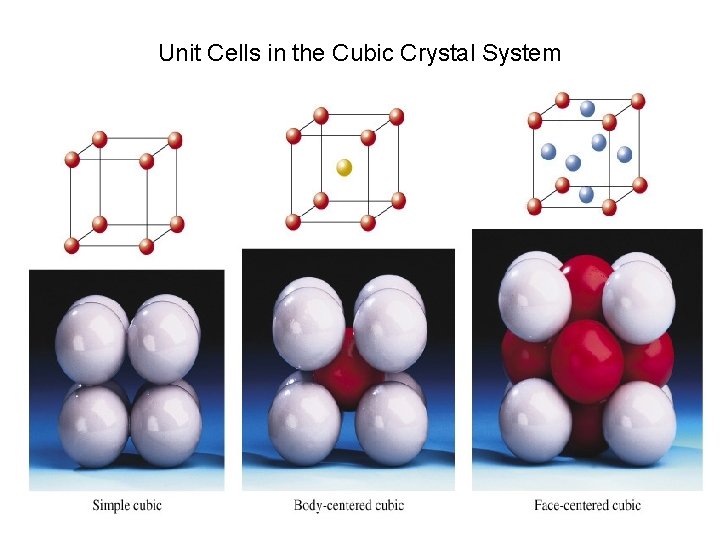
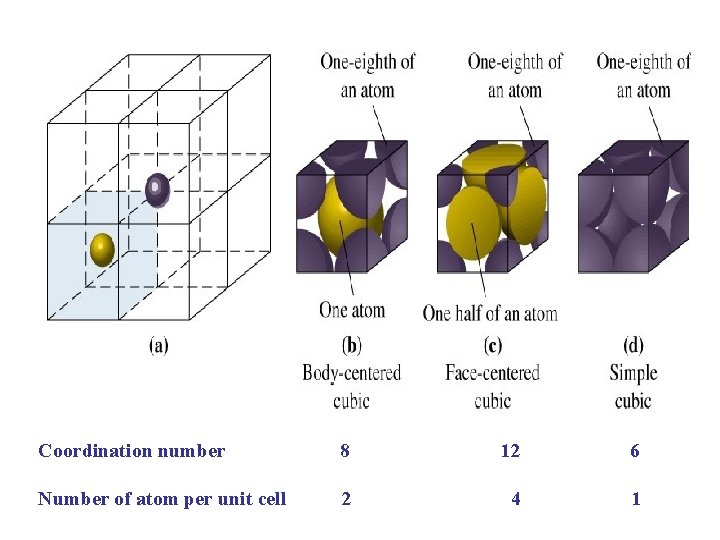
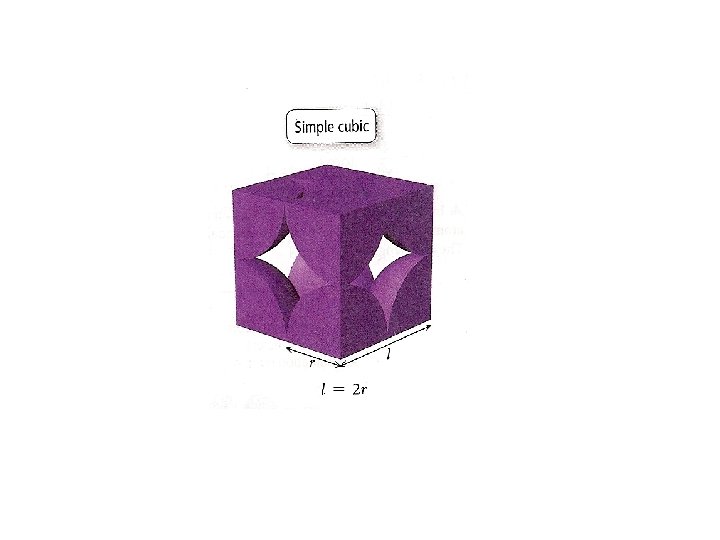
Unit Cells in the Cubic Crystal System



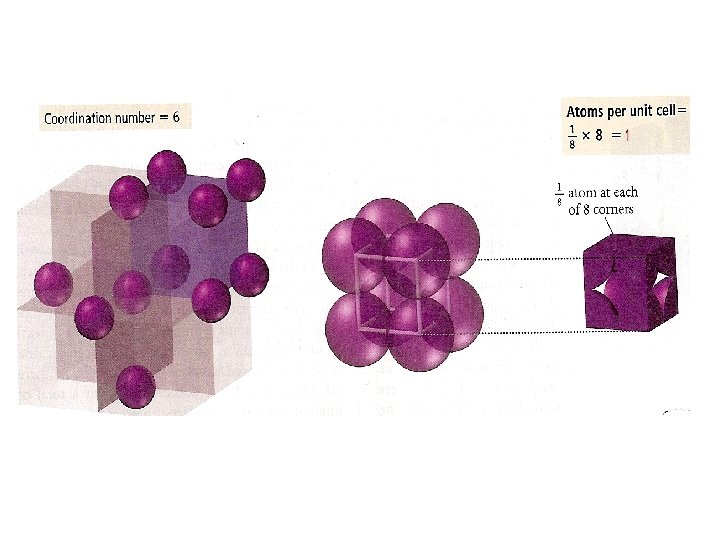
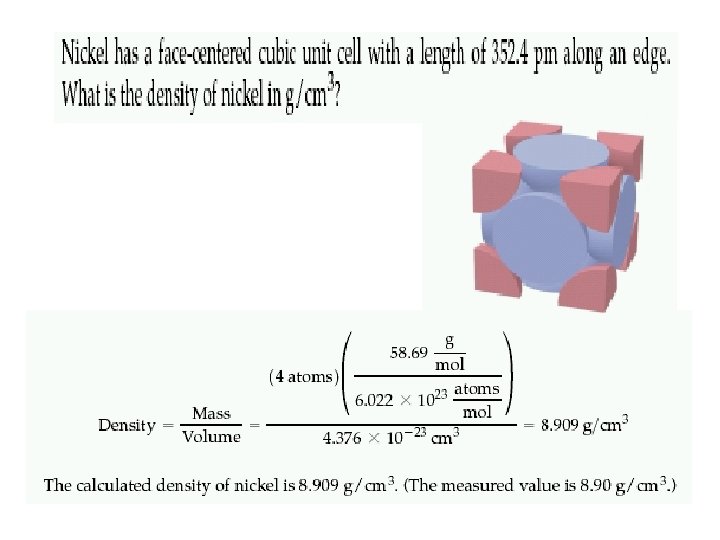
Coordination number 8 12 6 Number of atom per unit cell 2 4 1




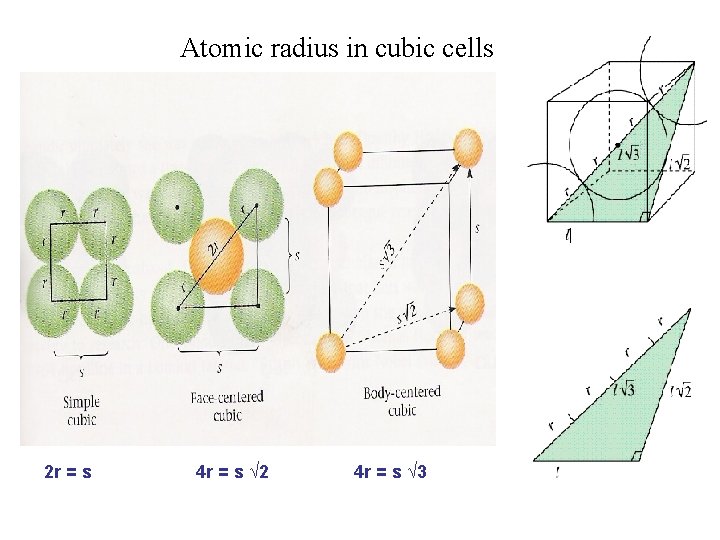
Atomic radius in cubic cells 2 r = s 4 r = s √ 2 4 r = s √ 3




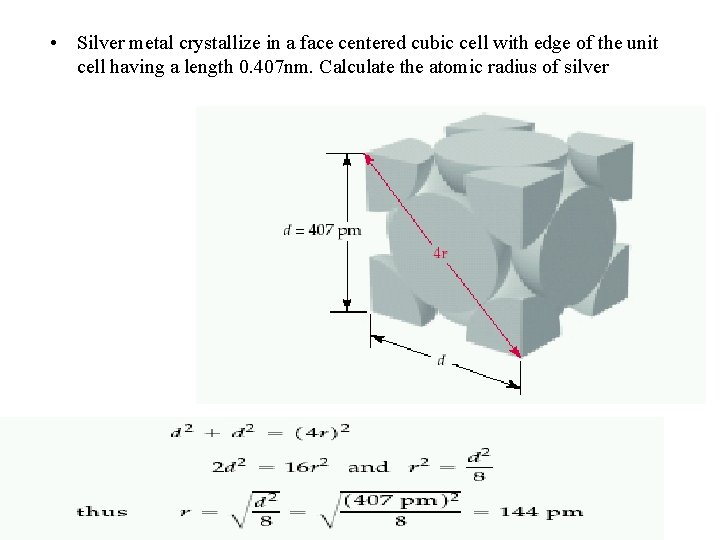
• Silver metal crystallize in a face centered cubic cell with edge of the unit cell having a length 0. 407 nm. Calculate the atomic radius of silver





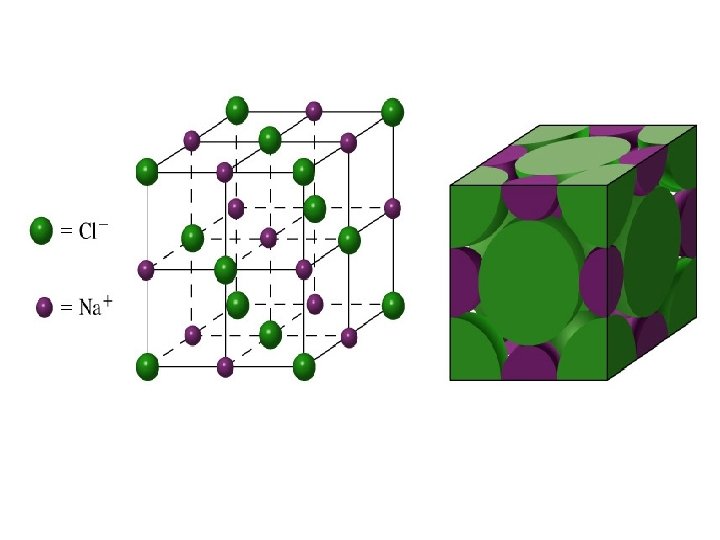
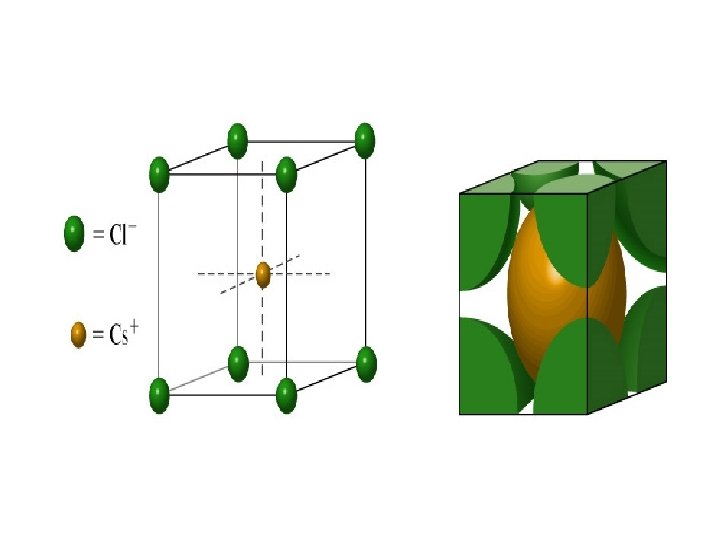
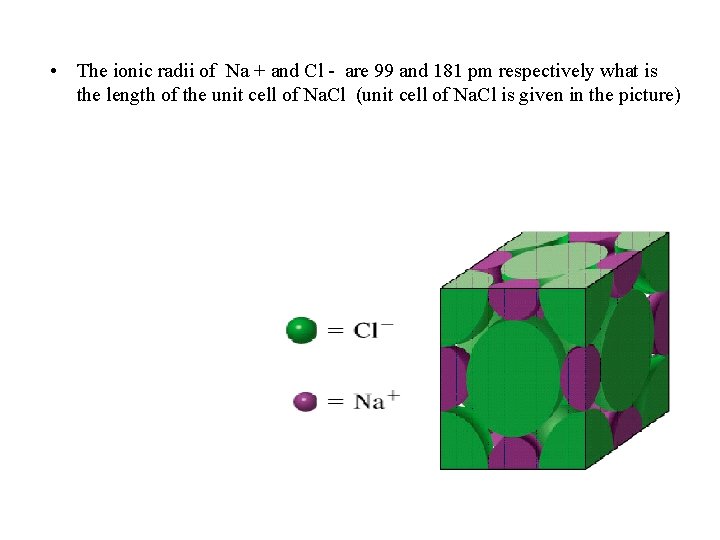
• The ionic radii of Na + and Cl - are 99 and 181 pm respectively what is the length of the unit cell of Na. Cl (unit cell of Na. Cl is given in the picture)

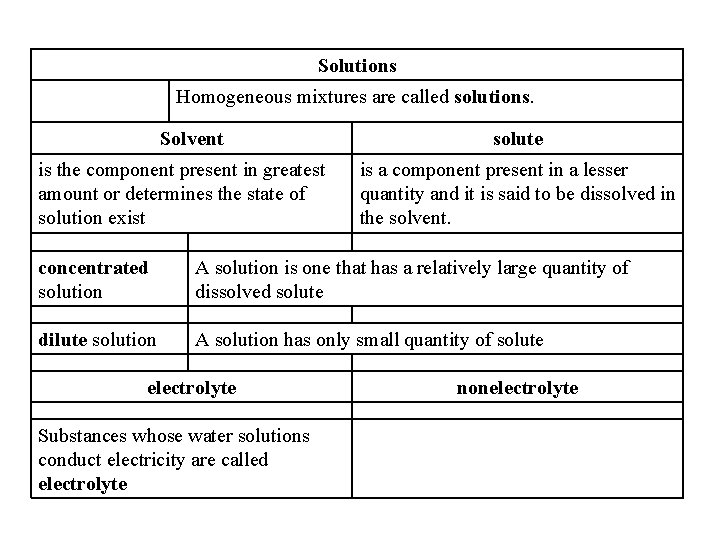
Solutions Homogeneous mixtures are called solutions. Solvent is the component present in greatest amount or determines the state of solution exist solute is a component present in a lesser quantity and it is said to be dissolved in the solvent. concentrated solution A solution is one that has a relatively large quantity of dissolved solute dilute solution A solution has only small quantity of solute electrolyte Substances whose water solutions conduct electricity are called electrolyte nonelectrolyte

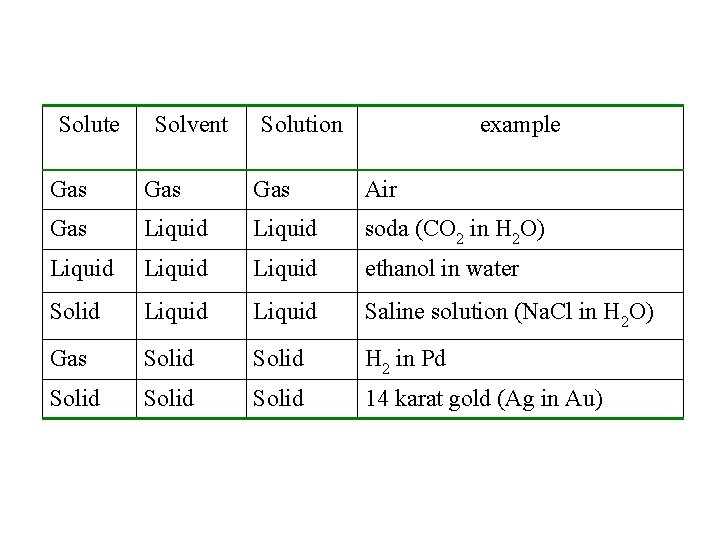
Solute Solvent Solution example Gas Gas Air Gas Liquid soda (CO 2 in H 2 O) Liquid ethanol in water Solid Liquid Saline solution (Na. Cl in H 2 O) Gas Solid H 2 in Pd Solid 14 karat gold (Ag in Au)

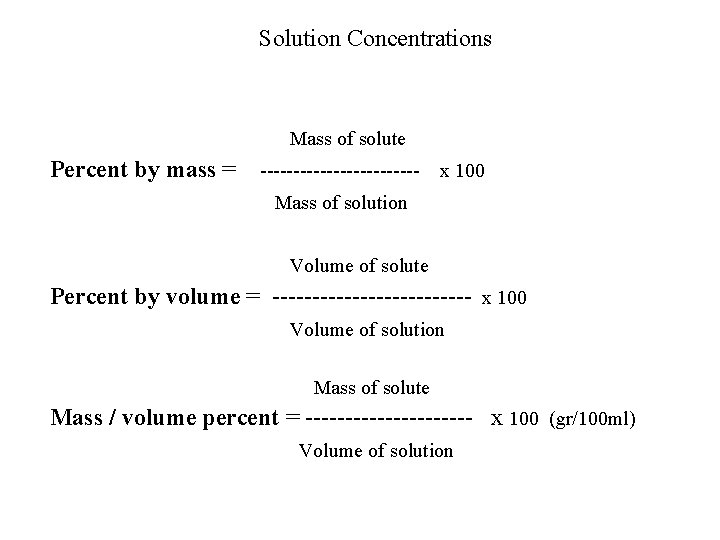
Solution Concentrations Mass of solute Percent by mass = ------------ x 100 Mass of solution Volume of solute Percent by volume = ------------- x 100 Volume of solution Mass of solute Mass / volume percent = ----------- x 100 (gr/100 ml) Volume of solution

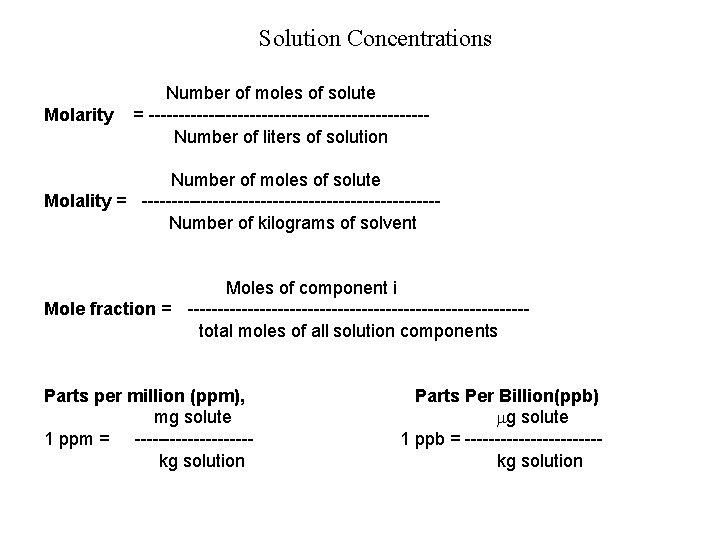
Solution Concentrations Molarity Number of moles of solute = -----------------------Number of liters of solution Number of moles of solute Molality = -------------------------Number of kilograms of solvent Moles of component i Mole fraction = ----------------------------total moles of all solution components Parts per million (ppm), mg solute 1 ppm = ----------kg solution Parts Per Billion(ppb) g solute 1 ppb = -----------kg solution

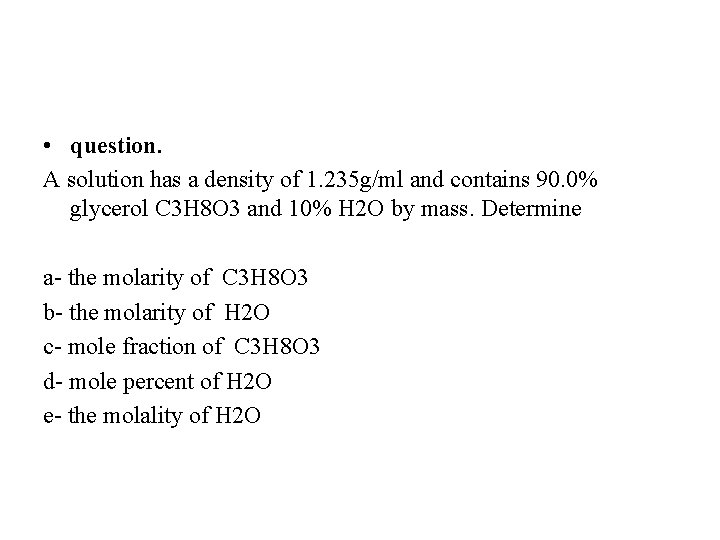
• question. A solution has a density of 1. 235 g/ml and contains 90. 0% glycerol C 3 H 8 O 3 and 10% H 2 O by mass. Determine a- the molarity of C 3 H 8 O 3 b- the molarity of H 2 O c- mole fraction of C 3 H 8 O 3 d- mole percent of H 2 O e- the molality of H 2 O

• question. A solution has a density of 1. 235 g/ml and contains 90. 0% glycerol C 3 H 8 O 3 and 10% H 2 O by mass. Determine a- the molarity of C 3 H 8 O 3 b- the molarity of H 2 O c- mole fraction of C 3 H 8 O 3 d- mole percent of H 2 O e- the molality of H 2 O