Solids and Liquids Chapter 14 Intermolecular forces Intramolecular








- Slides: 17

Solids and Liquids Chapter 14

Intermolecular forces Intramolecular forces • Bonding forces that hold atoms together within a molecule • The forces that occur among molecules that cause them to aggregate to form a solid or a liquid

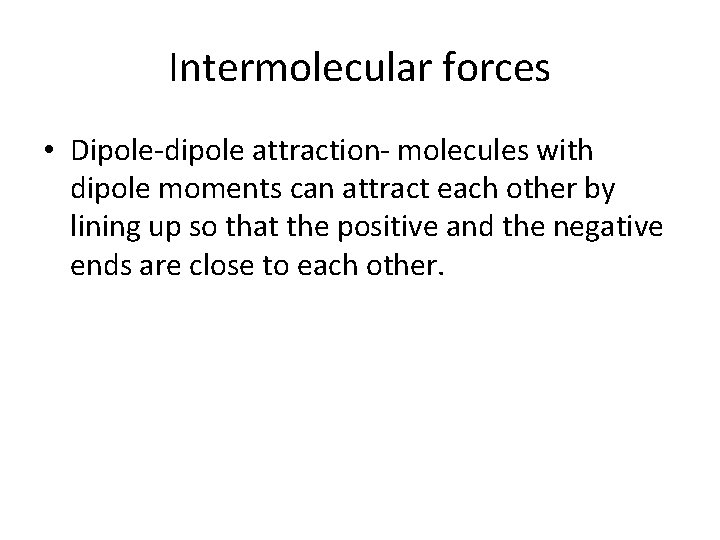
Intermolecular forces • Dipole-dipole attraction- molecules with dipole moments can attract each other by lining up so that the positive and the negative ends are close to each other.

• Hydrogen bonding- hydrogen is bound to two highly electronegative atoms, such as nitrogen, oxygen or fluorine.

• London dispersion forces- the forces that exist among noble gas atoms and nonpolar molecules

Solutions Chapter 15

Soluble vs. Insoluble Solubility (or Soluble) Insolubility (or insoluble) • Something that is capable of being dissolved in a solvent • Incapable of being dissolved within a given solution

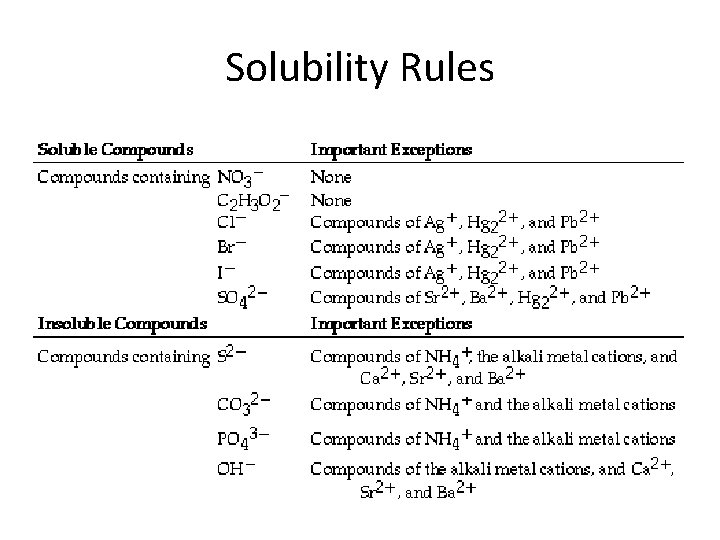
Solubility Rules

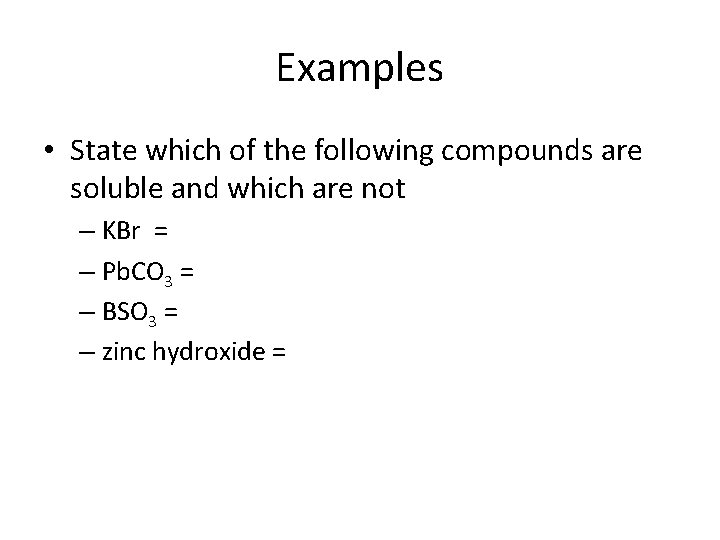
Examples • State which of the following compounds are soluble and which are not – KBr = – Pb. CO 3 = – BSO 3 = – zinc hydroxide =

– KBr = Soluble – Pb. CO 3 = Insoluble – BSO 3 =Insoluble – zinc hydroxide = Insoluble

Types of solutions • • • Solution-a homogeneous mixture Solvent-the largest substance that is present within the solution Solutes-the smallest substance within the solution Aqueous solution-solutions with water as a solvent Saturated-a solution that contains as much solute as will dissolve at the temperature Unsaturated- a solution that has not reached the limit of solute that will dissolve Concentrated-a large amount of solute dissolved Dilute-a relatively small amount of solute is dissolved Supersaturated- a solution that contains more dissolved material than could be dissolved by the solvent under normal circumstances

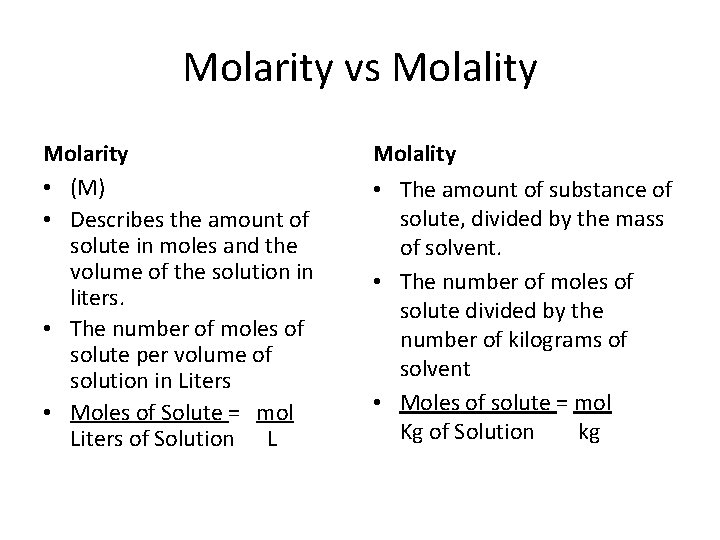
Molarity vs Molality Molarity • (M) • Describes the amount of solute in moles and the volume of the solution in liters. • The number of moles of solute per volume of solution in Liters • Moles of Solute = mol Liters of Solution L Molality • The amount of substance of solute, divided by the mass of solvent. • The number of moles of solute divided by the number of kilograms of solvent • Moles of solute = mol Kg of Solution kg

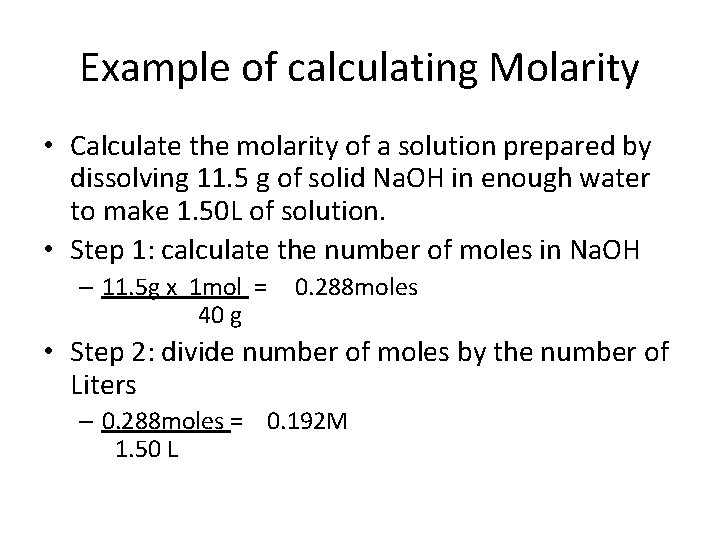
Example of calculating Molarity • Calculate the molarity of a solution prepared by dissolving 11. 5 g of solid Na. OH in enough water to make 1. 50 L of solution. • Step 1: calculate the number of moles in Na. OH – 11. 5 g x 1 mol = 40 g 0. 288 moles • Step 2: divide number of moles by the number of Liters – 0. 288 moles = 0. 192 M 1. 50 L

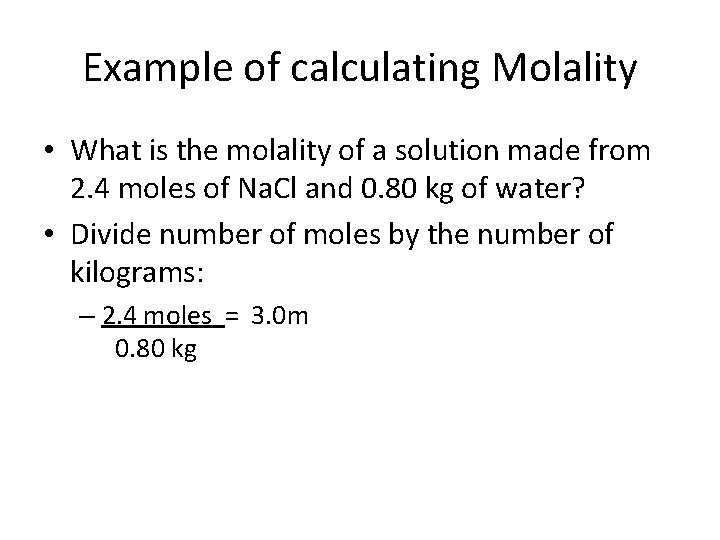
Example of calculating Molality • What is the molality of a solution made from 2. 4 moles of Na. Cl and 0. 80 kg of water? • Divide number of moles by the number of kilograms: – 2. 4 moles = 3. 0 m 0. 80 kg

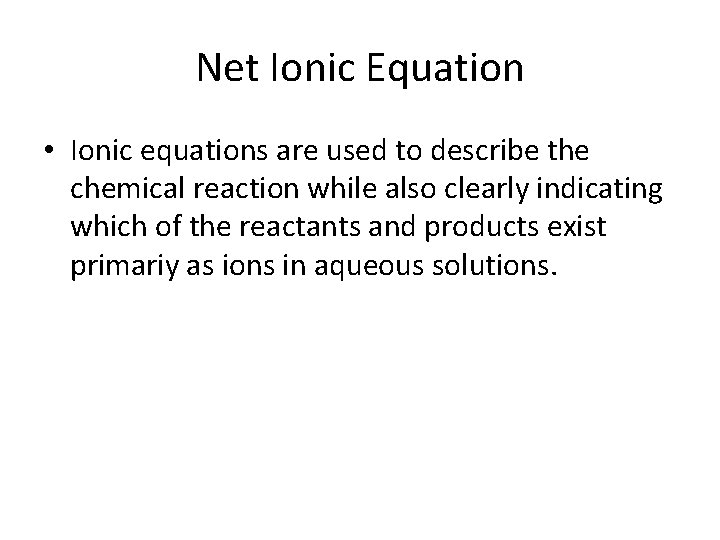
Net Ionic Equation • Ionic equations are used to describe the chemical reaction while also clearly indicating which of the reactants and products exist primariy as ions in aqueous solutions.

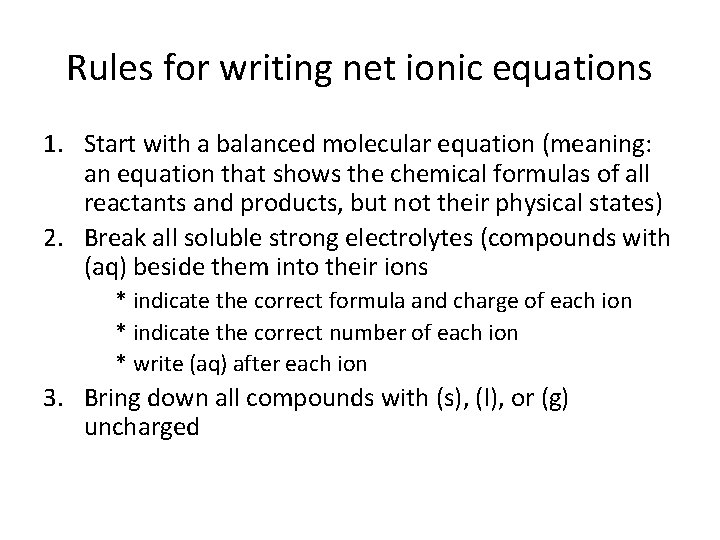
Rules for writing net ionic equations 1. Start with a balanced molecular equation (meaning: an equation that shows the chemical formulas of all reactants and products, but not their physical states) 2. Break all soluble strong electrolytes (compounds with (aq) beside them into their ions * indicate the correct formula and charge of each ion * indicate the correct number of each ion * write (aq) after each ion 3. Bring down all compounds with (s), (l), or (g) uncharged

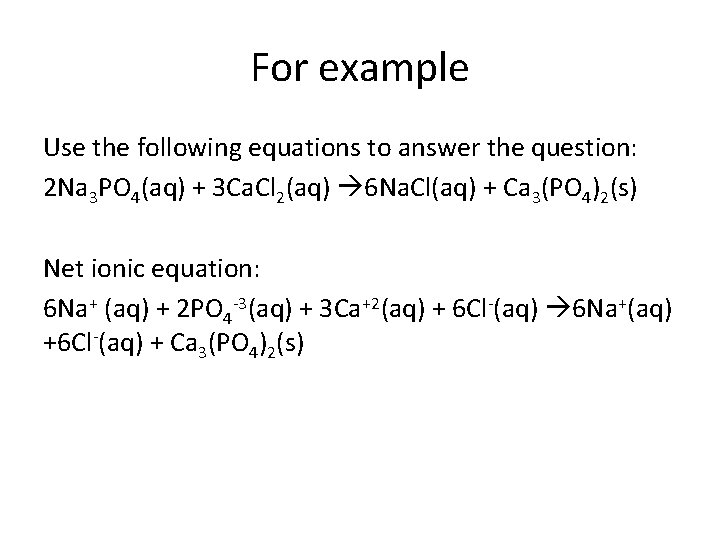
For example Use the following equations to answer the question: 2 Na 3 PO 4(aq) + 3 Ca. Cl 2(aq) 6 Na. Cl(aq) + Ca 3(PO 4)2(s) Net ionic equation: 6 Na+ (aq) + 2 PO 4 -3(aq) + 3 Ca+2(aq) + 6 Cl-(aq) 6 Na+(aq) +6 Cl-(aq) + Ca 3(PO 4)2(s)