Solids An Introduction to Structures and Types of













- Slides: 17

Solids An Introduction to Structures and Types of Solids

Types of Solids § The broadest categories of solids are: - Crystalline solids-those with a highly regular arrangement of their components. -Amorphous solids-those with considerable disorder in their structures

Components of Crystalline Solids § Lattice-a three-dimensional system of points designating the positions of the atoms, ions, or molecules § Unit cell-the smallest repeating unit of the lattice § See page 432 for the common unit cells and their lattices

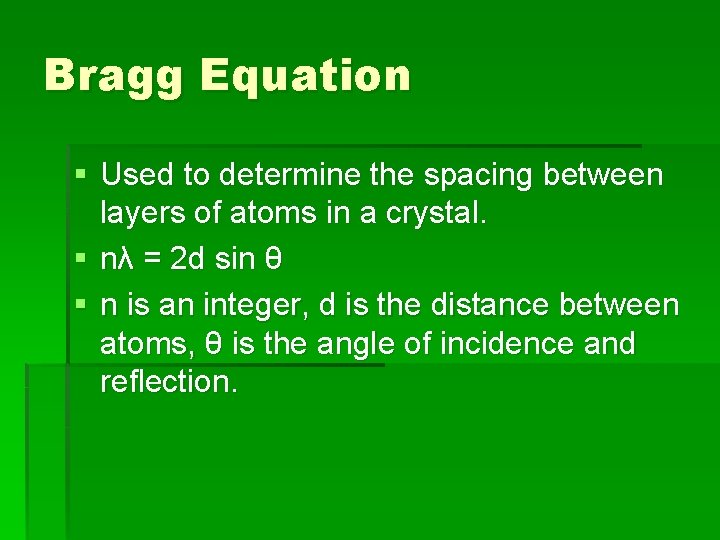
Bragg Equation § Used to determine the spacing between layers of atoms in a crystal. § nλ = 2 d sin θ § n is an integer, d is the distance between atoms, θ is the angle of incidence and reflection.

Example Problem Using the Bragg Equation § Example problem: X-rays of wavelength 1. 54 angstrom were used to analyze an aluminum crystal. A reflection was produced at θ = 19. 3 o. Assuming n=1, calculate the distance between the planes of atoms producing this reflection. § Answer: 233 pm

Types of Crystalline Solids § Ionic Solids-ions are at the points of the lattice. Ex: Sodium chloride, Na. Cl § Molecular Solids-covalently bonded molecules at the points of the lattice Ex: Ice, H 2 O molecules are at each point § Atomic Solids-atoms are at the points of the lattice. Ex: graphite (carbon at the lattice points)

Types of Atomic Solids § Metallic Solids- a special type of delocalized nondirectional covalent bonding occurs (this results in metals acting as good conductors of electricity) § Network Solids- nonmetallic atoms bond to each other with strong directional covalent bonds that lead to giant molecules, or networks, of atoms (example: carbon and silicon)

Types of Atomic Solids (continued) § Group 8 A Solids-the noble gases are attracted to each other with London dispersion forces. § For a summary, see the Table 10. 7 on page 458.

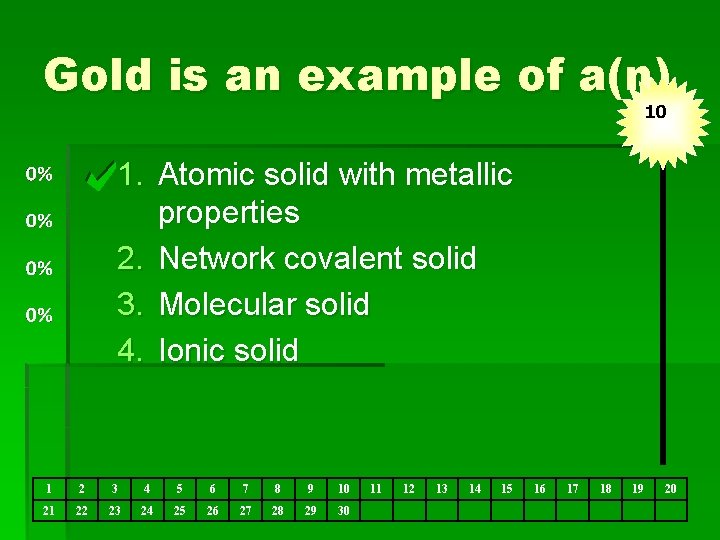
Gold is an example of a(n) 10 1. Atomic solid with metallic properties 2. Network covalent solid 3. Molecular solid 4. Ionic solid 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Carbon dioxide is an example of a(n) 1. 2. 3. 4. 10 Atomic solid Network covalent solid Molecular solid Ionic solid 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

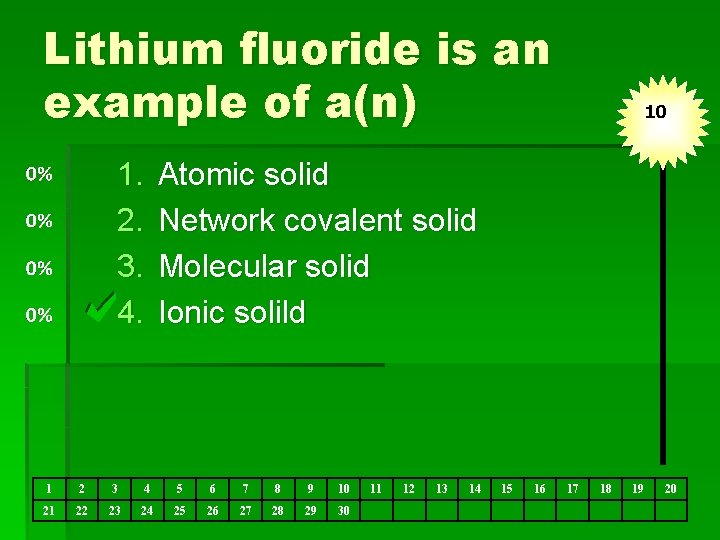
Lithium fluoride is an example of a(n) 1. 2. 3. 4. 10 Atomic solid Network covalent solid Molecular solid Ionic solild 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

Krypton is an example of a(n) 10 1. 2. 3. 4. Atomic solid Network covalent solid Molecular solid Ionic solid 1 2 3 4 5 6 7 8 9 10 21 22 23 24 25 26 27 28 29 30 11 12 13 14 15 16 17 18 19 20

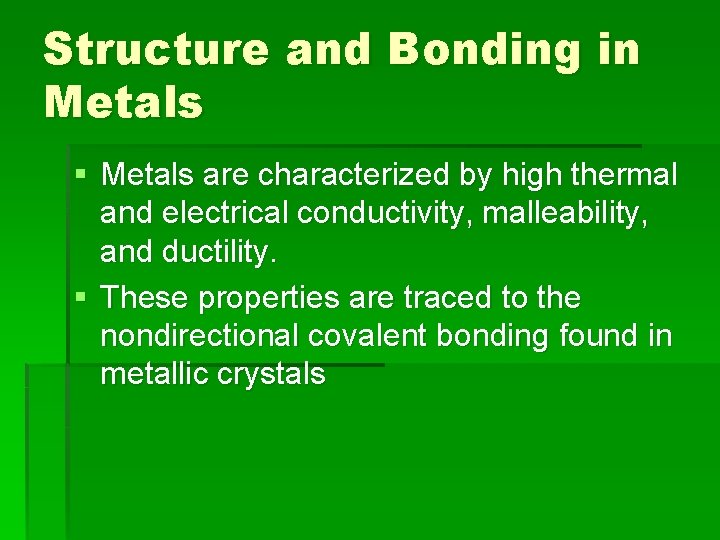
Structure and Bonding in Metals § Metals are characterized by high thermal and electrical conductivity, malleability, and ductility. § These properties are traced to the nondirectional covalent bonding found in metallic crystals

Types of Metallic Crystals § Hexagonal Closest Packing-the spherical metal atoms are packed in layers in which each sphere is surrounded by 6 others. § In the second layer, the spheres do not lie directly over those in the first layer. § Instead, each occupies an indentation formed by three spheres in the first layer. § This arrangement is commonly referred to as an ABA arrangement. § Examples of metals that form hexagonal closest packing are Mg and Zn.

Types of Metallic Crystals (continued) § Cubic Closest Packing- the first two layers are the same as the hexagonal closest packing. § In the fourth layer, the spheres occupy the same vertical position (instead of the third) § This arrangement is commonly referred to as the ABC arrangement. § In the ABC arrangement, the unit cell is face centered. § Examples of metals that form cubic closest packing are Al, Fe, Cu, Co, and Ni.

Determining the Density of a Closest Packed Solid § Complete the practice problems on page 439

Determining the Number of Ions in a Unit Cell § See page 457