SOLIDS 10 3 SOLIDS How do the particles






- Slides: 10

SOLIDS 10. 3

SOLIDS How do the particles in a solid behave? Shape and Volume Definite Melting Point Melting – the physical change of a solid to a liquid by the addition of energy as heat Melting point – the temp where solid becomes a liquid

SOLIDS Crystals – particles are arranged in an orderly, geometric, repeating pattern Crystalline solids – consists of crystals – Most Common Amorphous solid – particles arranged randomly Glass and plastics

PROPERTIES Amorphous substances have no definite melting pt Supercooled liquids – (aka amorphous substances) substances that retain certain liquid properties even at temps at which they appear to be solid

PROPERTIES High Density Low Compressibility Some seem compressible (wood and cork) but are not due to air pockets in the solid Low rate of diffusion

CRYSTALLINE SOLIDS Crystal structure – the 3 D arrangement of particles in a crystal Unit Cell – the smallest repeating pattern of a crystal lattice 7 different types of symmetry Pattern repeats in crystal

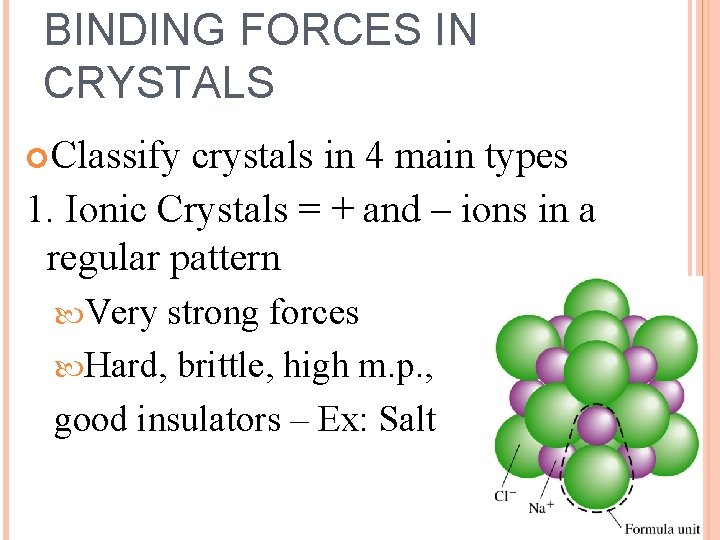
BINDING FORCES IN CRYSTALS Classify crystals in 4 main types 1. Ionic Crystals = + and – ions in a regular pattern Very strong forces Hard, brittle, high m. p. , good insulators – Ex: Salt

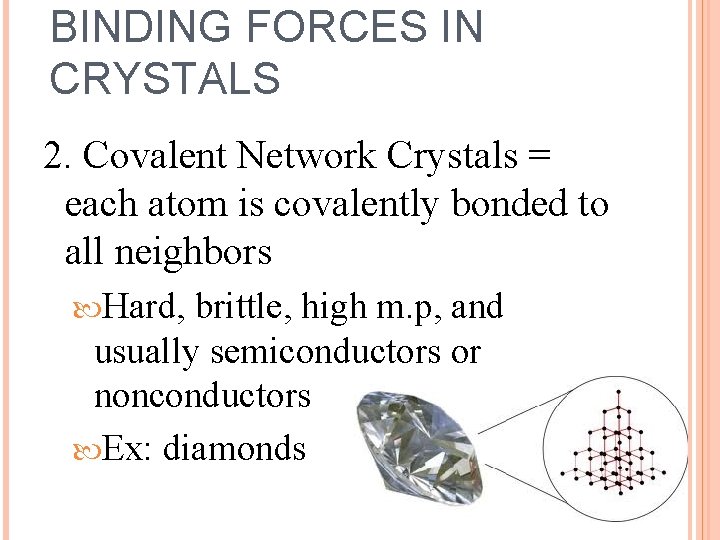
BINDING FORCES IN CRYSTALS 2. Covalent Network Crystals = each atom is covalently bonded to all neighbors Hard, brittle, high m. p, and usually semiconductors or nonconductors Ex: diamonds

BINDING FORCES IN CRYSTALS 3. Metallic Crystals = metal cations surrounded by valence e-’s Conductors Ex: Fe of electricity

BINDING FORCES IN CRYSTALS 4. Covalent molecular crystals = nonpolar molecules held together by London dispersion forces Low m. p. , easily vaporized, soft and good insulators – Ex: CO 2