Solid oxide devices based on protonconducting electrolytes prospects

Solid oxide devices based on proton-conducting electrolytes: prospects and problems Panagiotis Tsiakaras 1, 2 and Anatoly Demin 1 1 Institute of High-Temperature Electrochemistry, Yekaterinburg, Russia Laboratory of electrochemical devices based on solid oxide proton-conducting electrolytes 2 University of Thessaly, Volos, Greece

What is solid electrolyte? Solid material in which there is possibility for ions’ movement without destroy of its structure There a lot of solid materials with conductivity by cations and anions. What is solid oxide electrolyte? Solid oxide or solid oxide composition in which there is possibility for oxygen ions or protons movement

Necessary conditions for oxygen conductivity in solid oxides 2 -D model of a crystal of АО 2 oxide 2 - 2 - + 2 - 2 - 4+ 2 - 2 - 2 - 2 - 2 - 2 - 4+ 2 - 2 - 2 - 4+ 2 - 2 - 4+ 4+ 2 - 24+ 2 - 2 - 2 - 4+ 4+ 4+ 2 - 2 - 2 - 2 - 2 - 4+ 4+ 2 - 2 - 4+ 4+ 2 - 2 - 2 - 24+ 4+ 4+ 2 - 2 - 2 - 24+ 2 - 2 - 2 -

2 -D model of a solid solutions АО 2 + ВО A part of АО 2 molecules is substituted by ВО molecules Some positions in an anion sublattice are empty (vacancies). Oxygen ions can jump from normal position to an empty one. 2 - 2 - 2 - 4+ 2 - 2 - 2 - 2 - 2 - 24+ 2 - 2 - 2 - 4+ 2+ 2 - 2 - 4+ 2 - 24+ 2 - 2 - 2 - 24+ 4+ 2+ 2 - 2 - 4+ 4+ 4+ 2 - 2 - 4+ 2 - 24+ 4+ 4+ 2 - 24+ 2+ 2 - 2 - 2 - 4+ 2 - 2 -
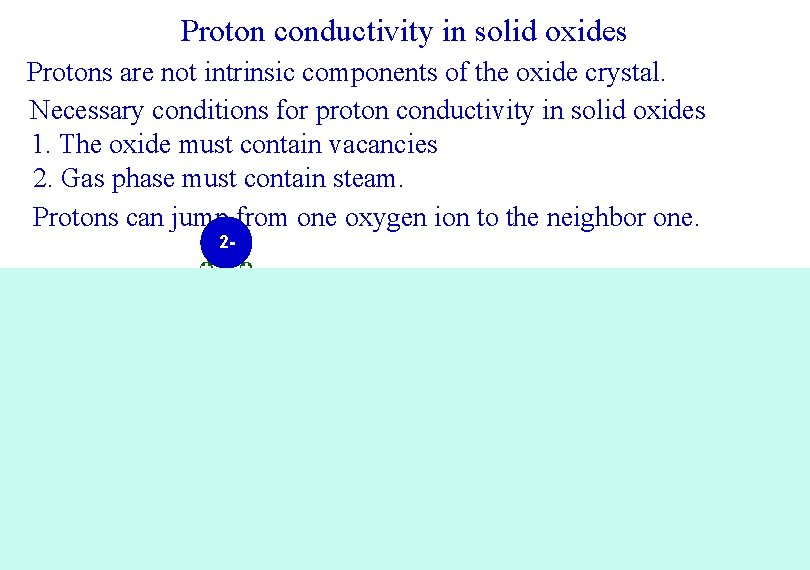
Proton conductivity in solid oxides Protons are not intrinsic components of the oxide crystal. Necessary conditions for proton conductivity in solid oxides 1. The oxide must contain vacancies 2. Gas phase must contain steam. Protons can jump from one oxygen ion to the neighbor one. 2 - 2 - 24+ 2 - 4+ 2+ 2 - 2 - 2 - 24+ 2 - 2 - 4+ 2 - 24+ 2 -

Solid oxide electrolyte cell based on oxygen ion electrolyte proton conducting electrolyte Bound О 2 (Н 2+H 2 O) O 2 - Free Н 2 O E Free О 2 E stands for electromotive force (also called “open circuit voltage”, OCV) H+ Bound Н 2 (H 2 O+О 2) H E
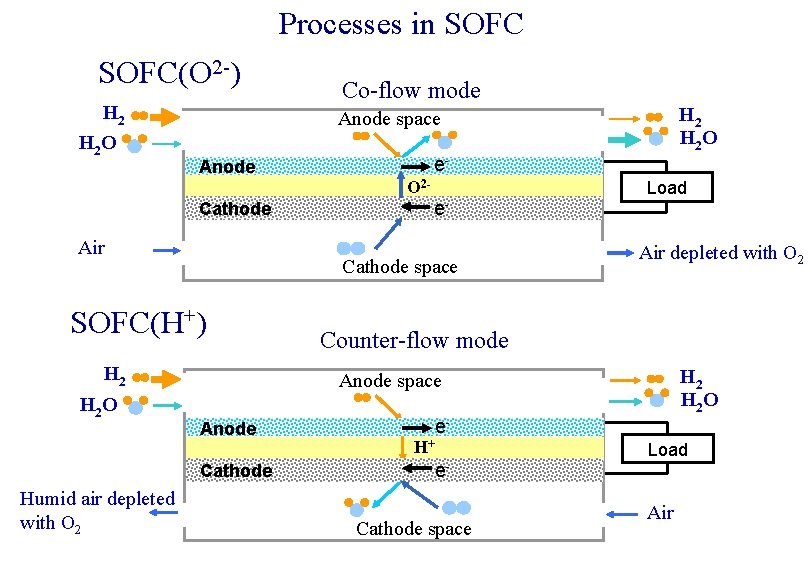
Processes in SOFC(O 2 -) H 2 O Co-flow mode e- Anode O 2 Cathode Air H 2 Load Air depleted with O 2 Counter-flow mode H 2 O Anode space Anode Cathode Humid air depleted with O 2 e- Cathode space SOFC(Н+) H 2 O Anode space e. Н+ e. Cathode space Load Air
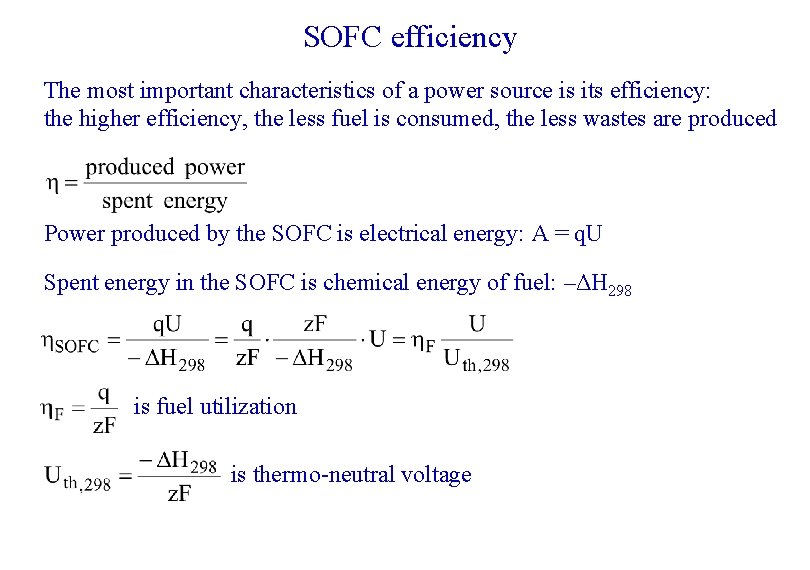
SOFC efficiency The most important characteristics of a power source is its efficiency: the higher efficiency, the less fuel is consumed, the less wastes are produced Power produced by the SOFC is electrical energy: А = q. U Spent energy in the SOFC is chemical energy of fuel: –ΔН 298 is fuel utilization is thermo-neutral voltage
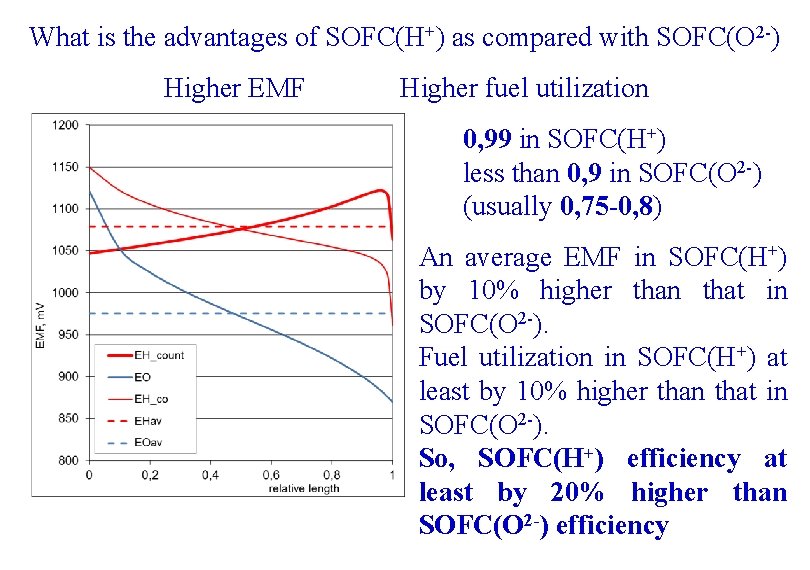
What is the advantages of SOFC(H+) as compared with SOFC(O 2 -) Higher EMF Higher fuel utilization 0, 99 in SOFC(H+) less than 0, 9 in SOFC(O 2 -) (usually 0, 75 -0, 8) An average EMF in SOFC(H+) by 10% higher than that in SOFC(O 2 -). Fuel utilization in SOFC(H+) at least by 10% higher than that in SOFC(O 2 -). So, SOFC(H+) efficiency at least by 20% higher than SOFC(O 2 -) efficiency
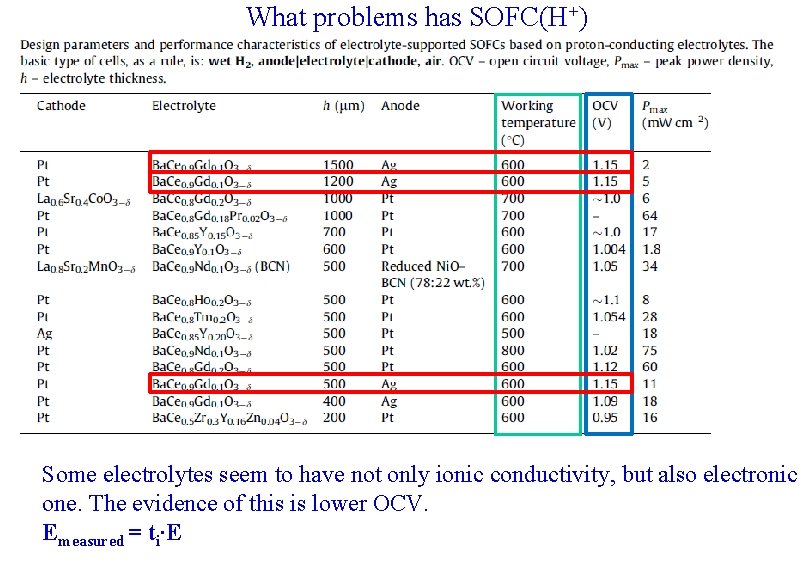
What problems has SOFC(H+) Some electrolytes seem to have not only ionic conductivity, but also electronic one. The evidence of this is lower OCV. Emeasured = ti∙E
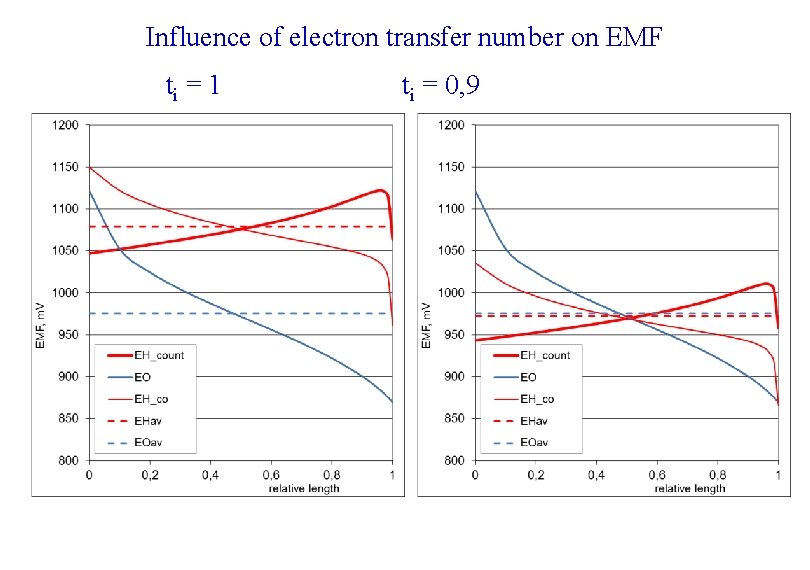
Influence of electron transfer number on EMF ti = 1 ti = 0, 9

The main task is to find the electrolytes with highest protonic conductivity and develop the technology for producing gas-tight electrolytes. Such electrolytes can be also the base for other electrochemical devices, namely i) solid oxide electrolyzers for producing high purity H 2; ii) sensors for analyzing hydrogen-containing gas mixtures; iii) devices for measuring diffusion coefficient of hydrogen in different gas mixtures.

Thank you for your attention!
- Slides: 13