Sol ids Phase diagrams and phase transitions of




- Slides: 50

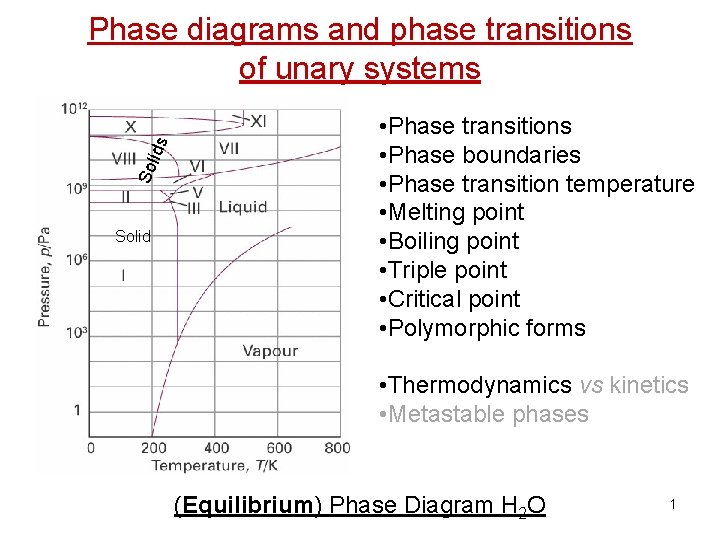
Sol ids Phase diagrams and phase transitions of unary systems Solid • Phase transitions • Phase boundaries • Phase transition temperature • Melting point • Boiling point • Triple point • Critical point • Polymorphic forms • Thermodynamics vs kinetics • Metastable phases (Equilibrium) Phase Diagram H 2 O 1

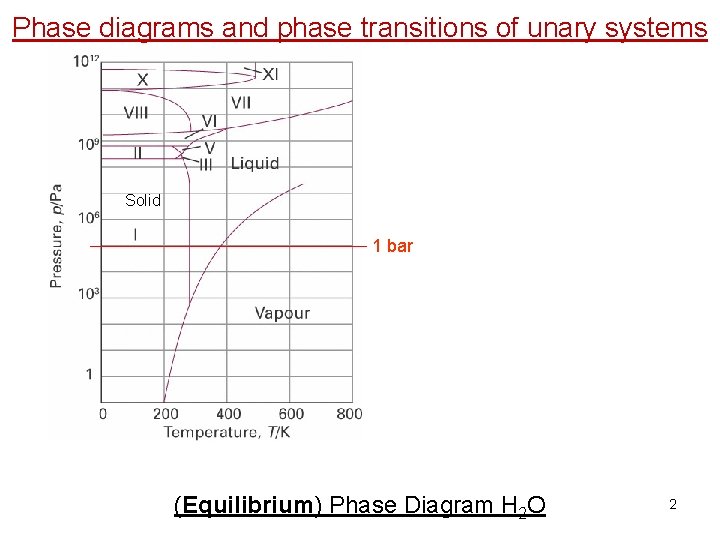
Phase diagrams and phase transitions of unary systems Solid 1 bar (Equilibrium) Phase Diagram H 2 O 2

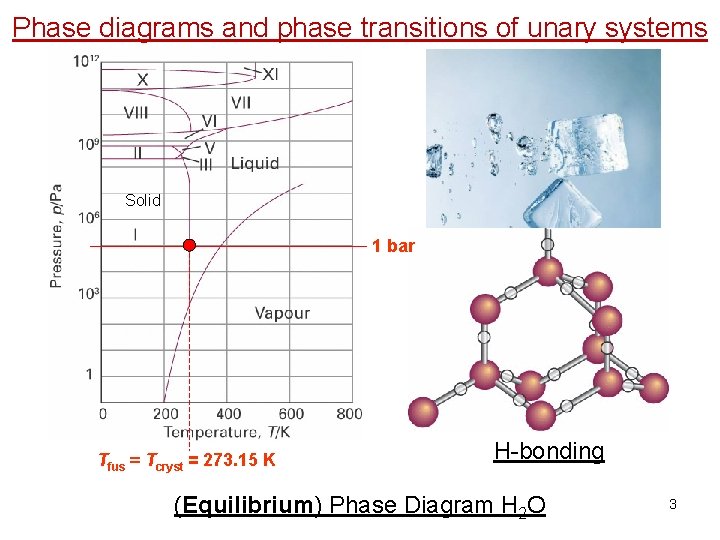
Phase diagrams and phase transitions of unary systems Solid 1 bar Tfus = Tcryst = 273. 15 K H-bonding (Equilibrium) Phase Diagram H 2 O 3

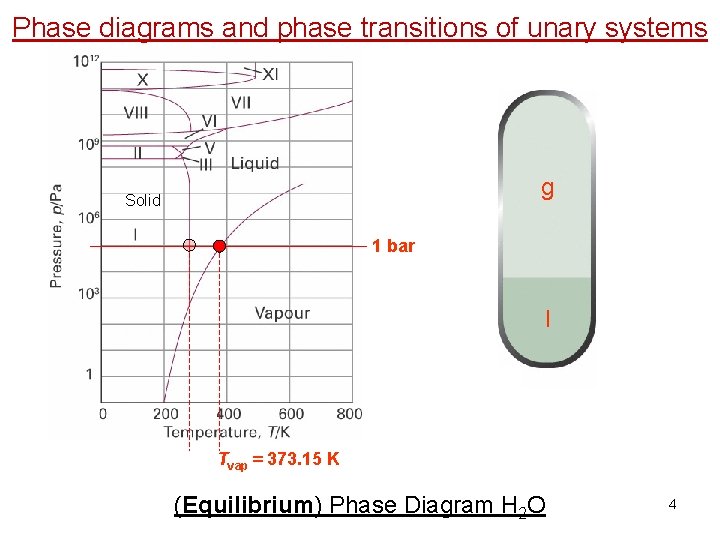
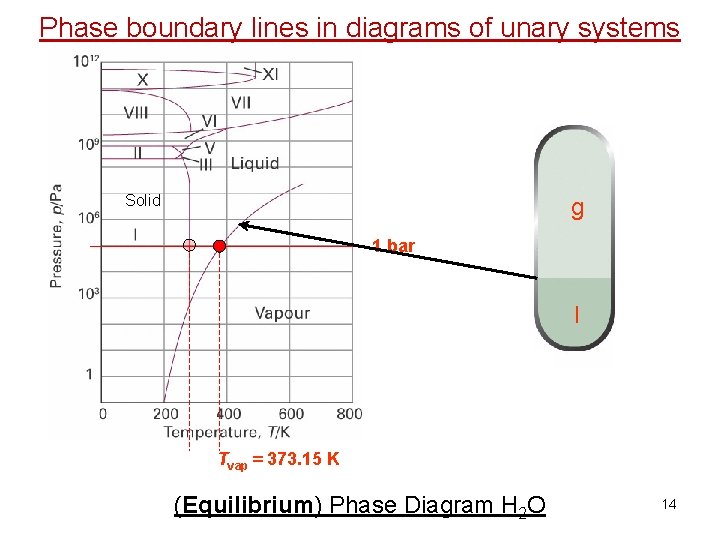
Phase diagrams and phase transitions of unary systems g Solid 1 bar l Tvap = 373. 15 K (Equilibrium) Phase Diagram H 2 O 4

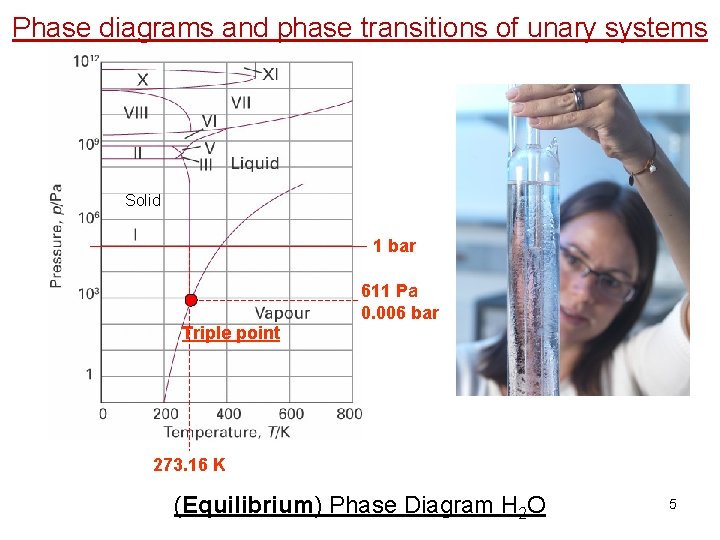
Phase diagrams and phase transitions of unary systems Solid 1 bar 611 Pa 0. 006 bar Triple point 273. 16 K (Equilibrium) Phase Diagram H 2 O 5

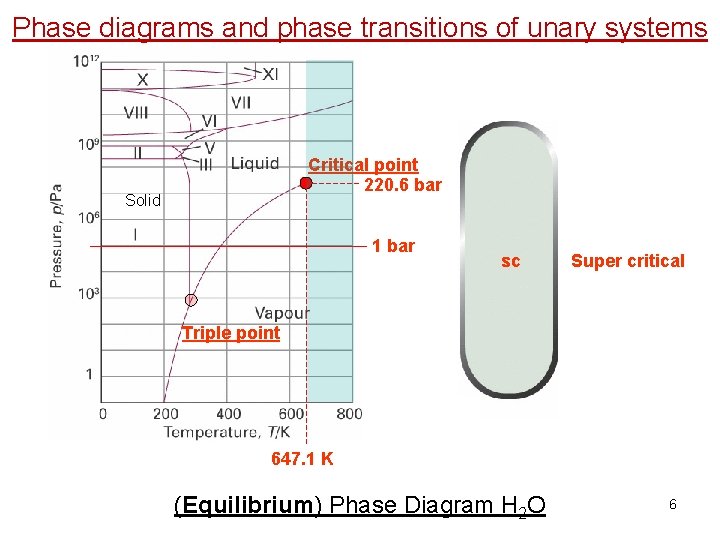
Phase diagrams and phase transitions of unary systems Critical point 220. 6 bar Solid 1 bar sc Super critical Triple point 647. 1 K (Equilibrium) Phase Diagram H 2 O 6

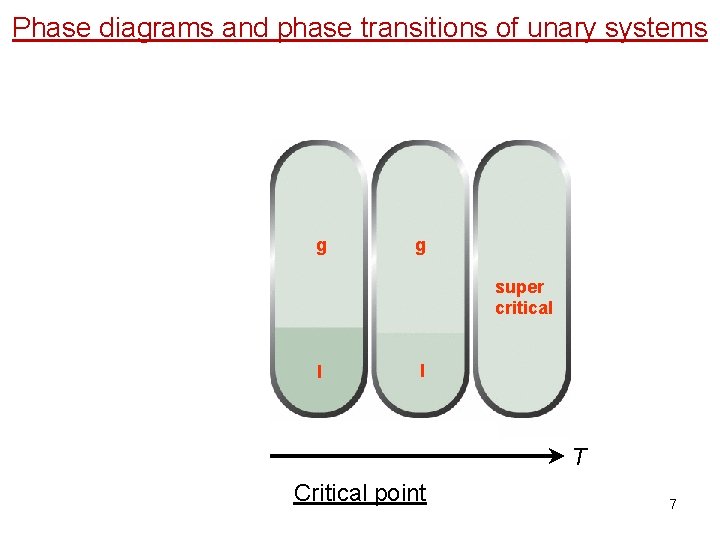
Phase diagrams and phase transitions of unary systems g g super critical l l T Critical point 7

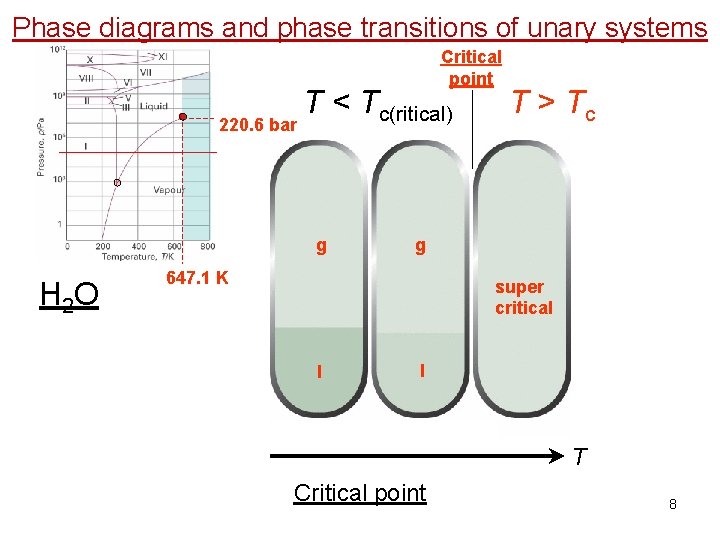
Phase diagrams and phase transitions of unary systems Critical point 220. 6 bar T < Tc(ritical) g H 2 O T > Tc g 647. 1 K super critical l l T Critical point 8

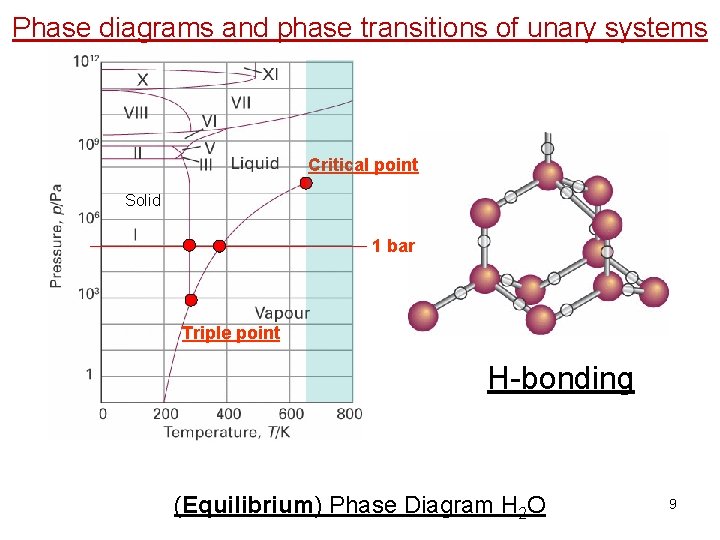
Phase diagrams and phase transitions of unary systems Critical point Solid 1 bar Triple point H-bonding (Equilibrium) Phase Diagram H 2 O 9

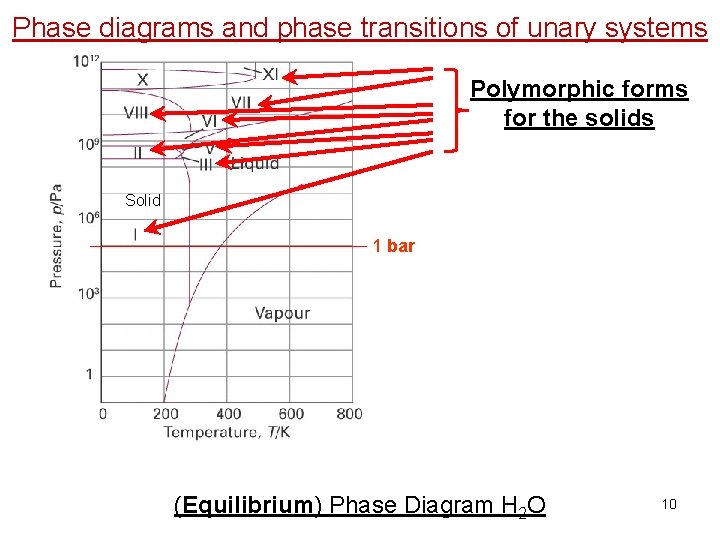
Phase diagrams and phase transitions of unary systems Polymorphic forms for the solids Solid 1 bar (Equilibrium) Phase Diagram H 2 O 10

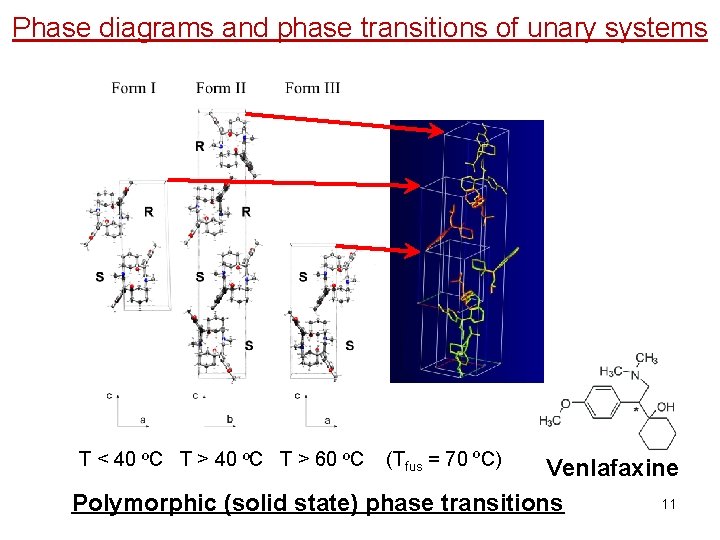
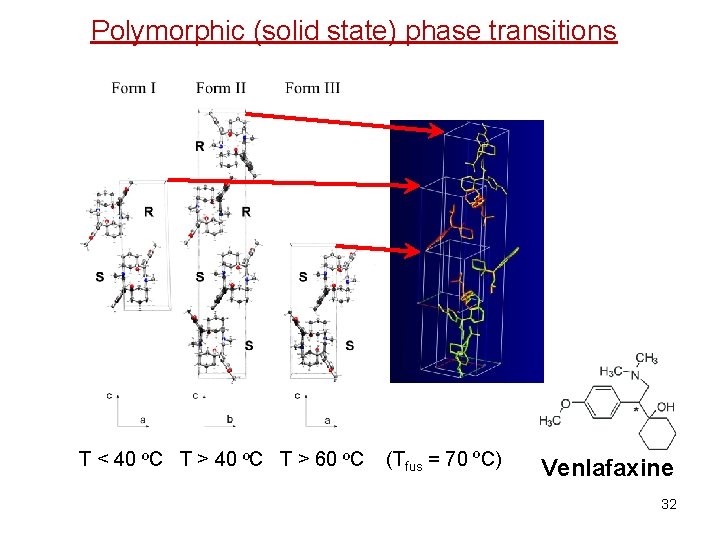
Phase diagrams and phase transitions of unary systems T < 40 ºC T > 60 ºC (Tfus = 70 ºC) Venlafaxine 11 Polymorphic (solid state) phase transitions

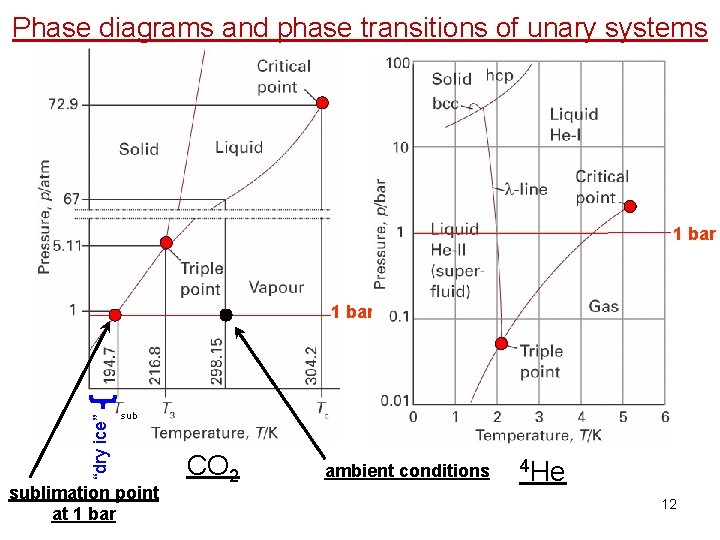
Phase diagrams and phase transitions of unary systems 1 bar “dry ice” 1 bar sublimation point at 1 bar CO 2 ambient conditions 4 He 12

Phase boundary lines in diagrams of unary systems 13

Phase boundary lines in diagrams of unary systems Solid g 1 bar l Tvap = 373. 15 K (Equilibrium) Phase Diagram H 2 O 14

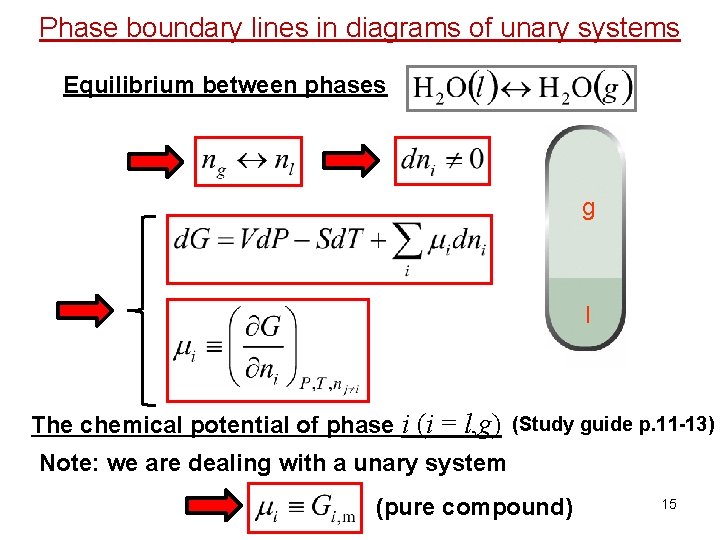
Phase boundary lines in diagrams of unary systems Equilibrium between phases g l The chemical potential of phase i (i = l, g) (Study guide p. 11 -13) Note: we are dealing with a unary system (pure compound) 15

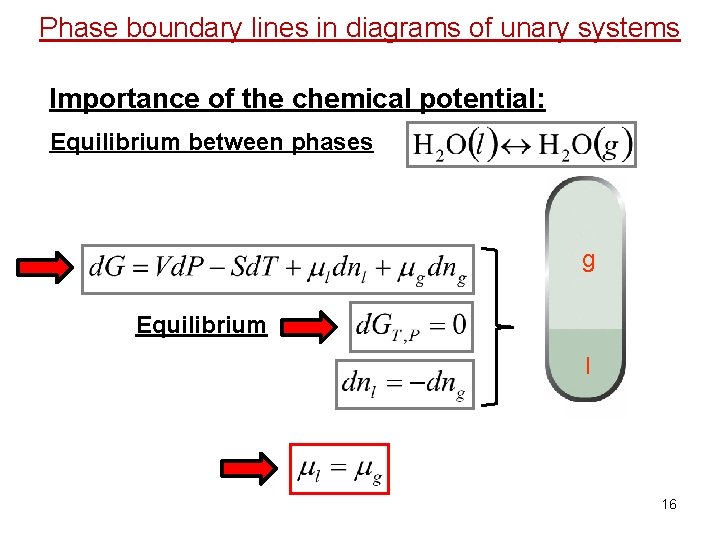
Phase boundary lines in diagrams of unary systems Importance of the chemical potential: Equilibrium between phases g Equilibrium l 16

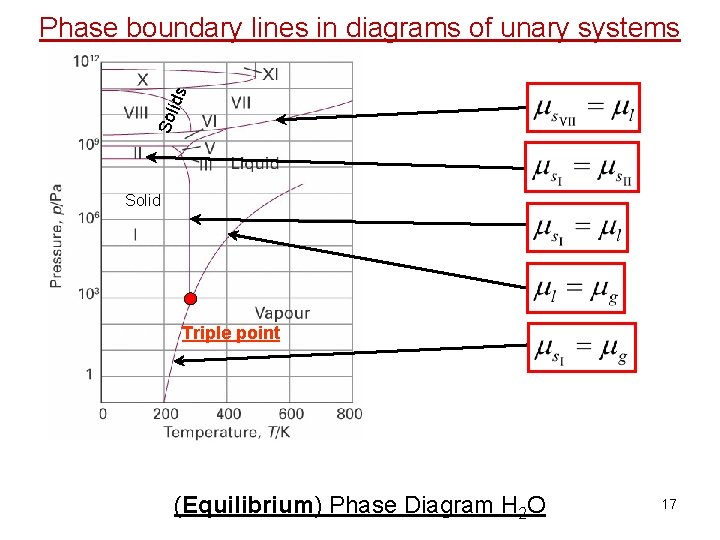
Sol ids Phase boundary lines in diagrams of unary systems Solid Triple point (Equilibrium) Phase Diagram H 2 O 17

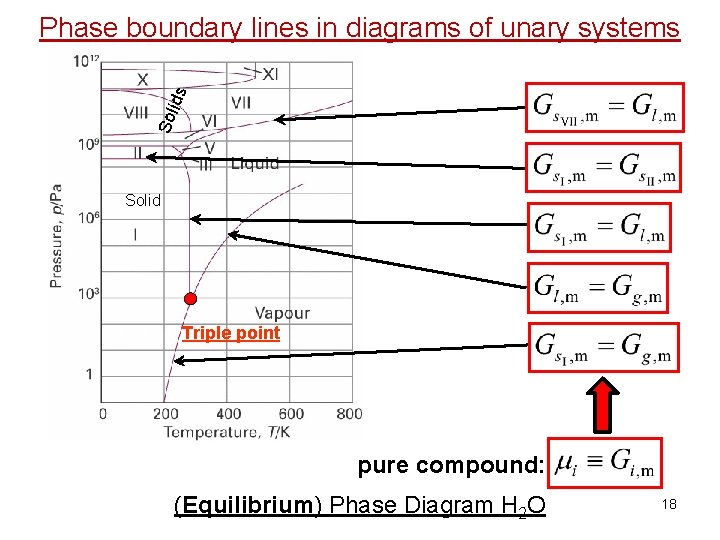
Sol ids Phase boundary lines in diagrams of unary systems Solid Triple point pure compound: (Equilibrium) Phase Diagram H 2 O 18

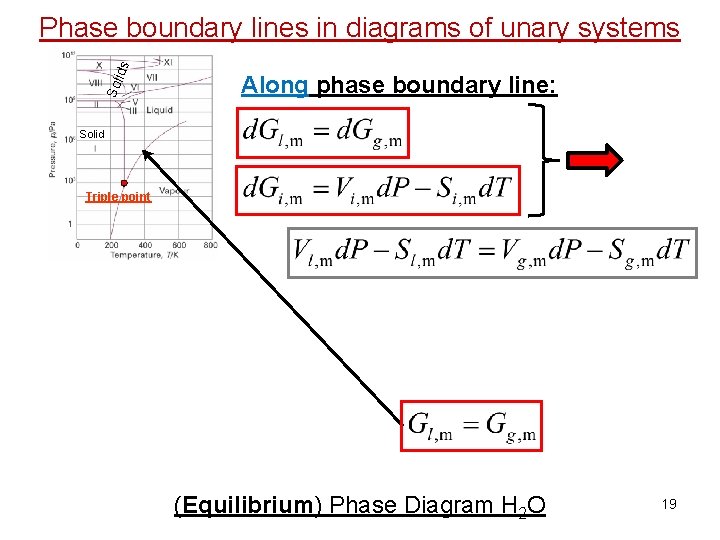
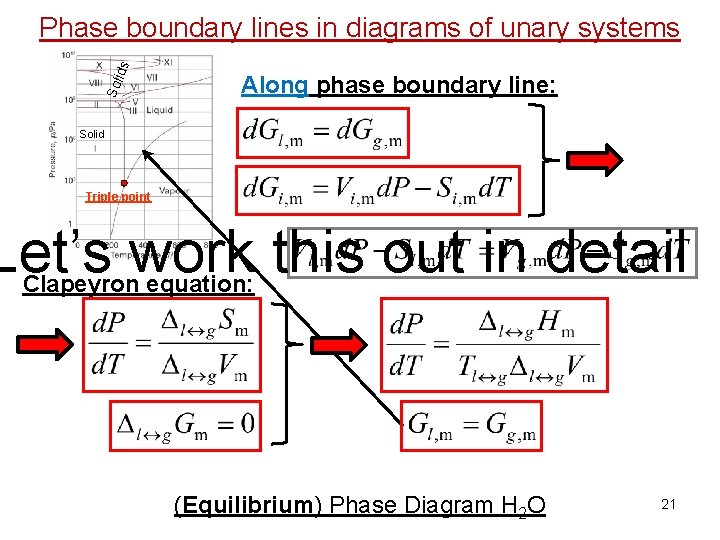
Sol id s Phase boundary lines in diagrams of unary systems Along phase boundary line: Solid Triple point (Equilibrium) Phase Diagram H 2 O 19

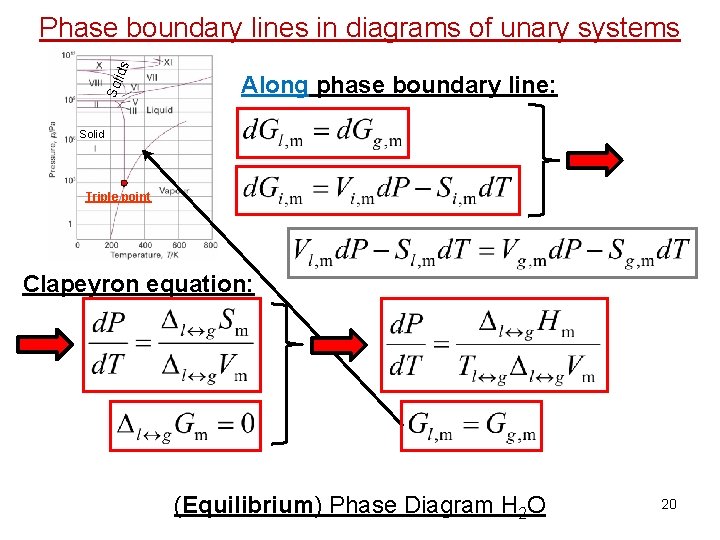
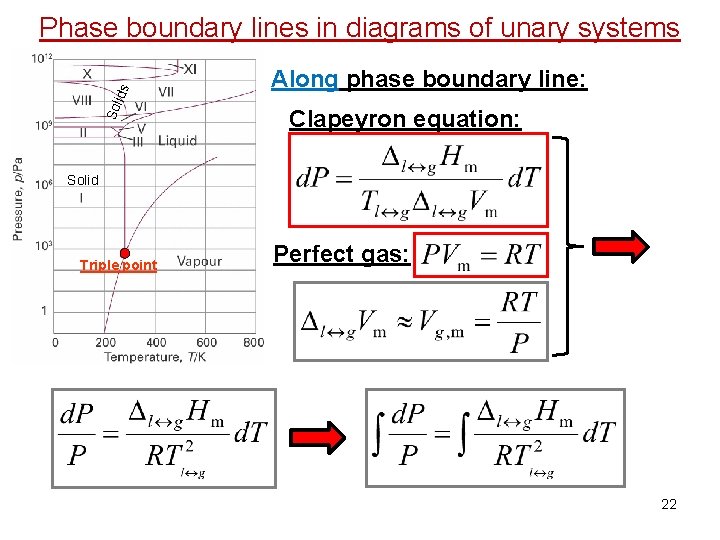
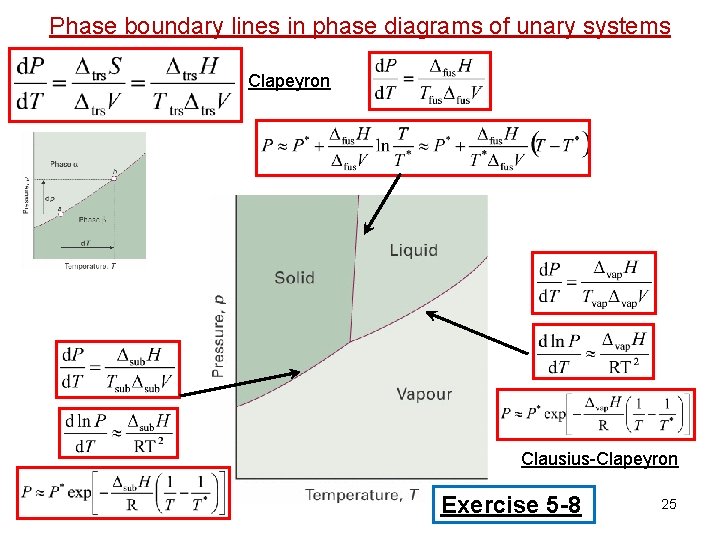
Sol id s Phase boundary lines in diagrams of unary systems Along phase boundary line: Solid Triple point Clapeyron equation: (Equilibrium) Phase Diagram H 2 O 20

Sol id s Phase boundary lines in diagrams of unary systems Along phase boundary line: Solid Triple point Let’s work this out in detail Clapeyron equation: (Equilibrium) Phase Diagram H 2 O 21

Sol id s Phase boundary lines in diagrams of unary systems Along phase boundary line: Clapeyron equation: Solid Triple point Perfect gas: 22

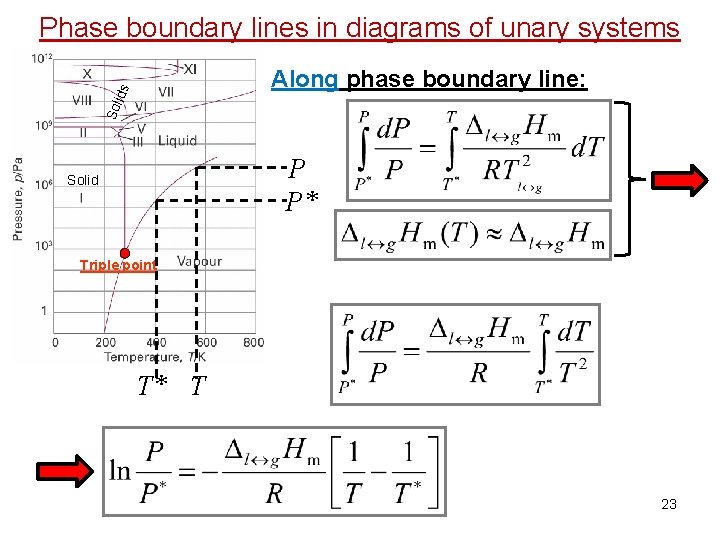
Phase boundary lines in diagrams of unary systems Sol id s Along phase boundary line: P P* Solid Triple point T* T 23

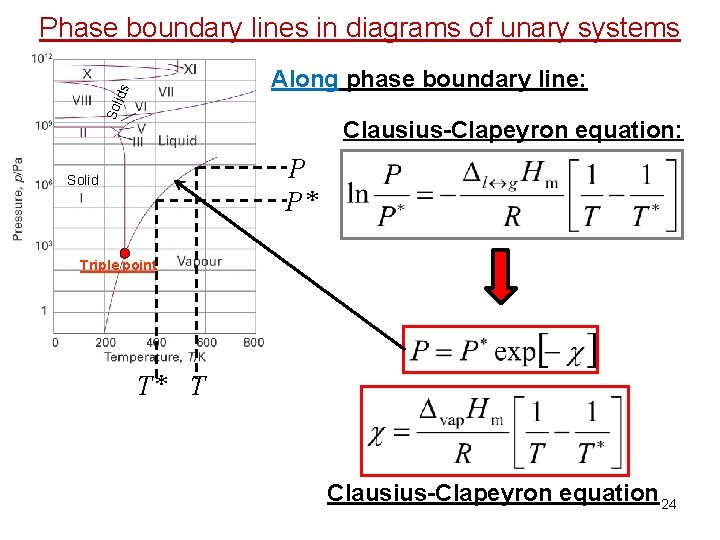
Phase boundary lines in diagrams of unary systems Sol id s Along phase boundary line: Clausius-Clapeyron equation: P P* Solid Triple point T* T Clausius-Clapeyron equation 24

Phase boundary lines in phase diagrams of unary systems Clapeyron Clausius-Clapeyron Exercise 5 -8 25

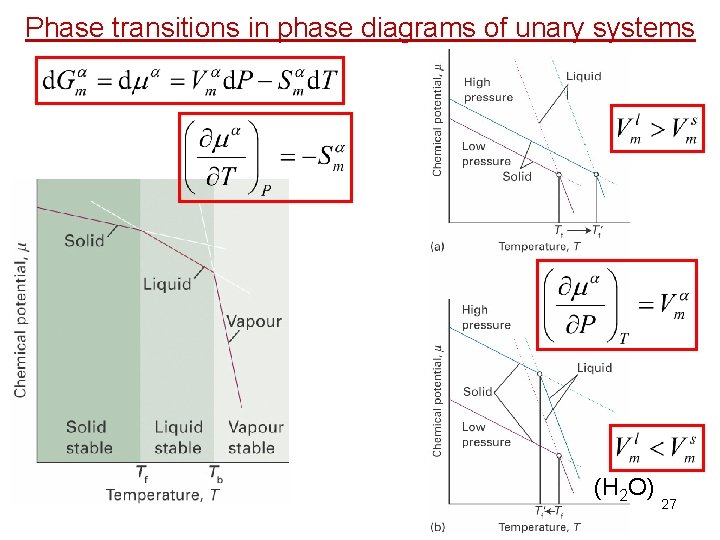
Phase transitions in phase diagrams of unary systems 26

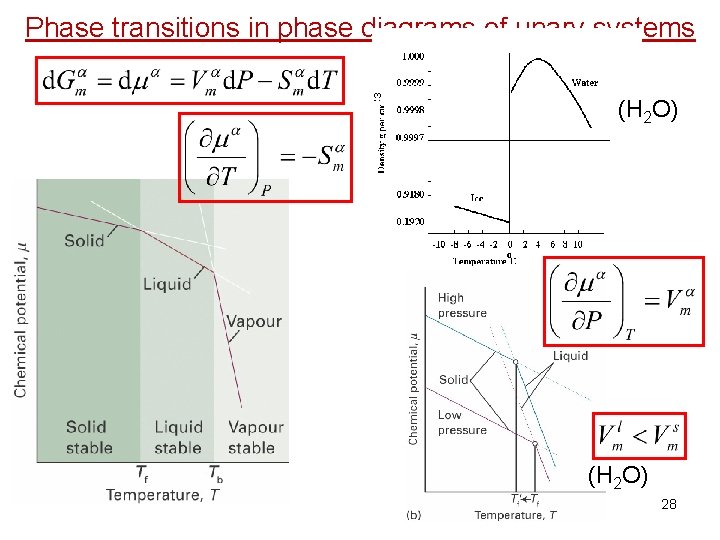
Phase transitions in phase diagrams of unary systems (H 2 O) 27

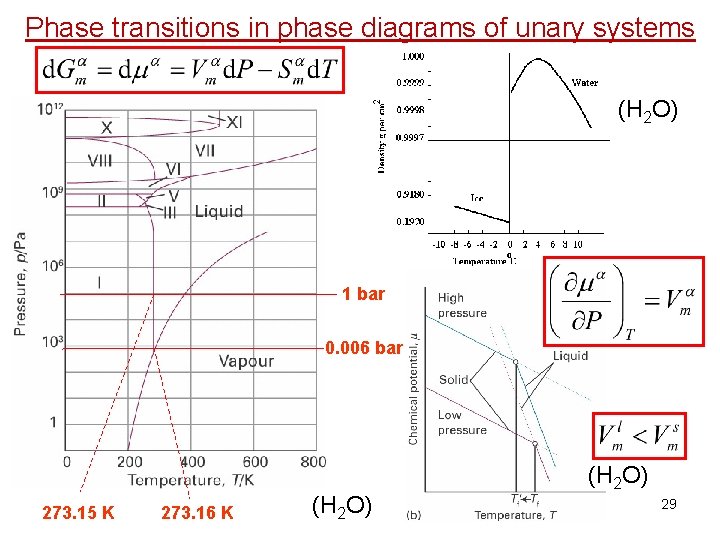
3 Phase transitions in phase diagrams of unary systems (H 2 O) 28

Phase transitions in phase diagrams of unary systems (H 2 O) 1 bar 0. 006 bar 273. 15 K 273. 16 K (H 2 O) 29

30

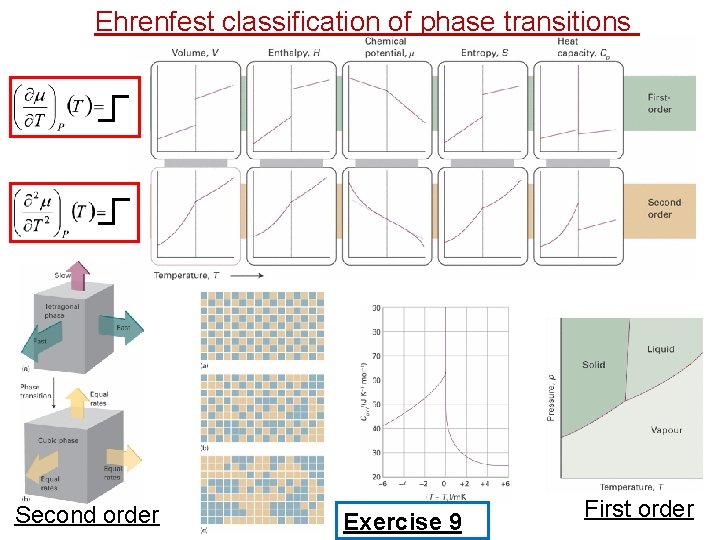
Ehrenfest classification of phase transitions Second order Exercise 9 First order

Polymorphic (solid state) phase transitions T < 40 ºC T > 60 ºC (Tfus = 70 ºC) Venlafaxine 32

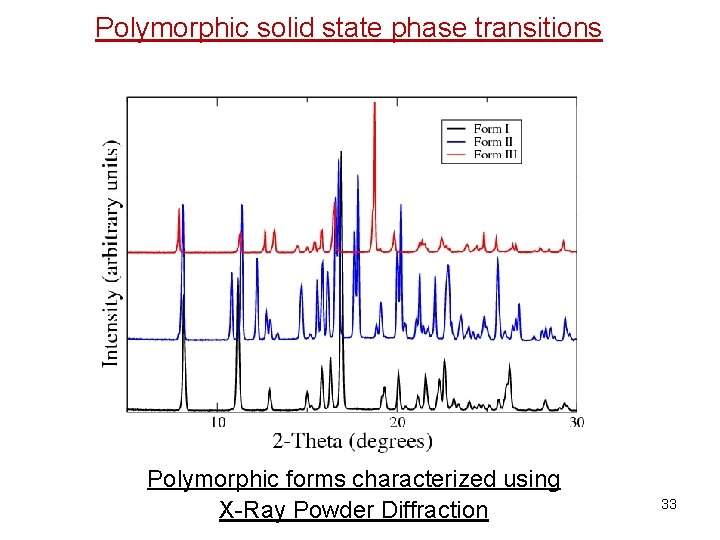
Polymorphic solid state phase transitions Polymorphic forms characterized using X-Ray Powder Diffraction 33

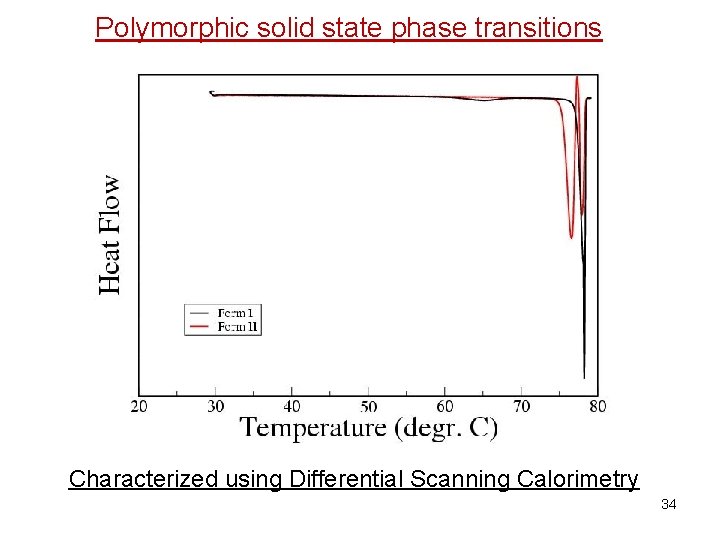
Polymorphic solid state phase transitions Characterized using Differential Scanning Calorimetry 34

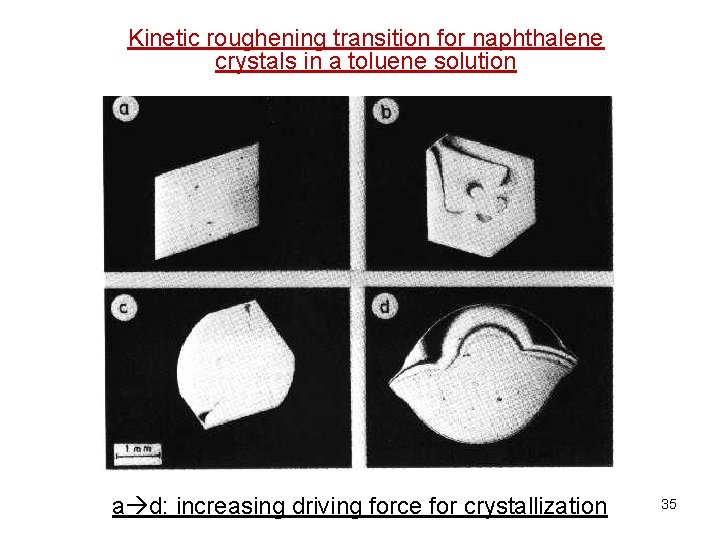
Kinetic roughening transition for naphthalene crystals in a toluene solution a d: increasing driving force for crystallization 35

Gibbs phase rule: multicomponent phases 36

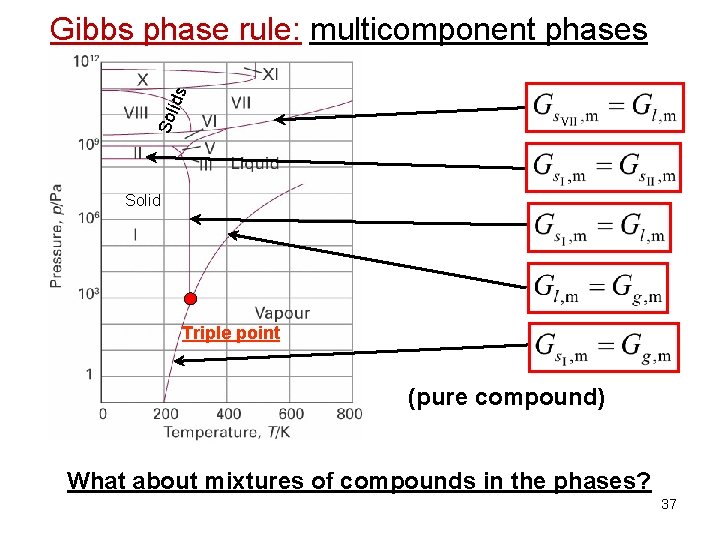
Sol ids Gibbs phase rule: multicomponent phases Solid Triple point (pure compound) What about mixtures of compounds in the phases? 37

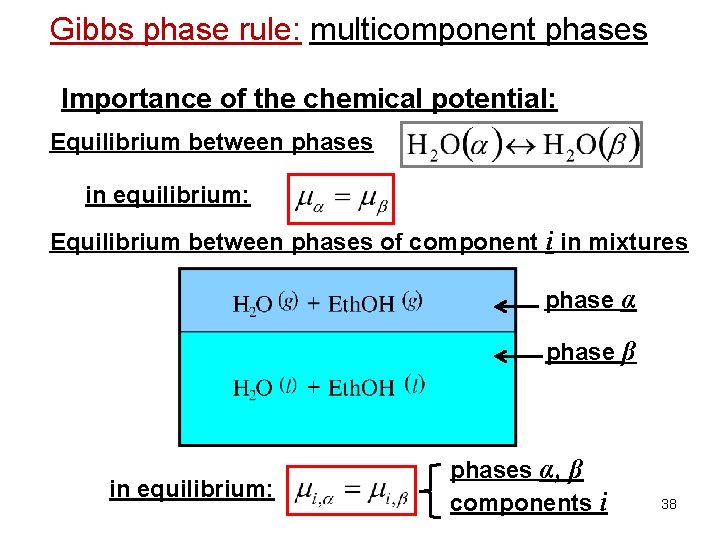
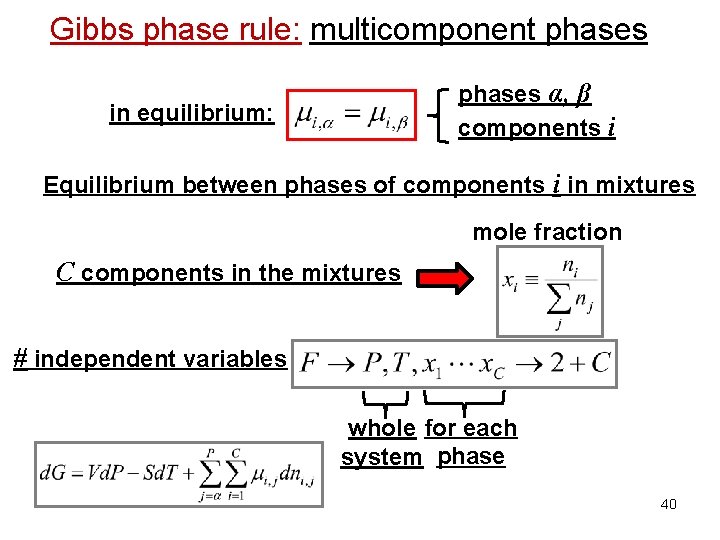
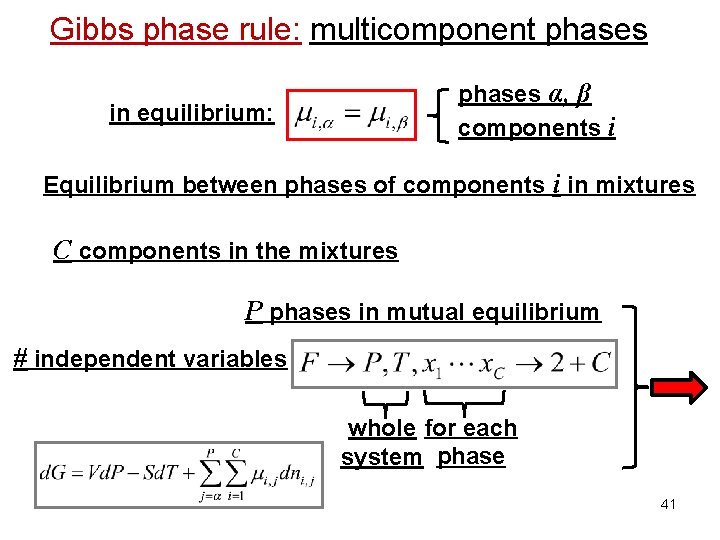
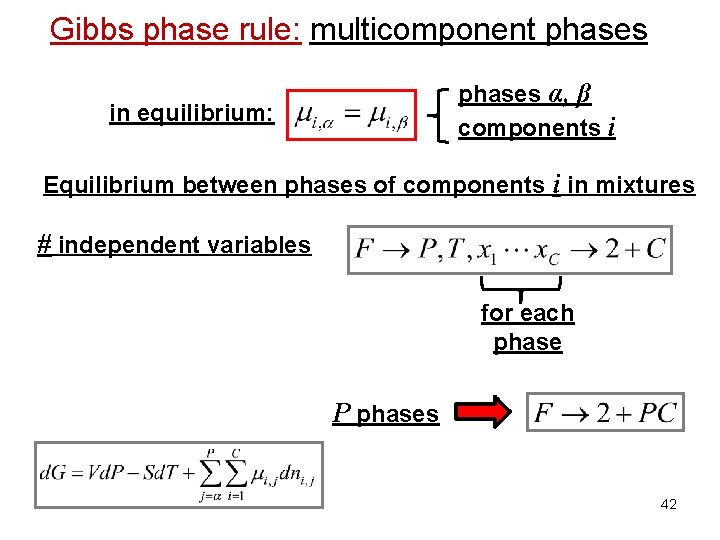
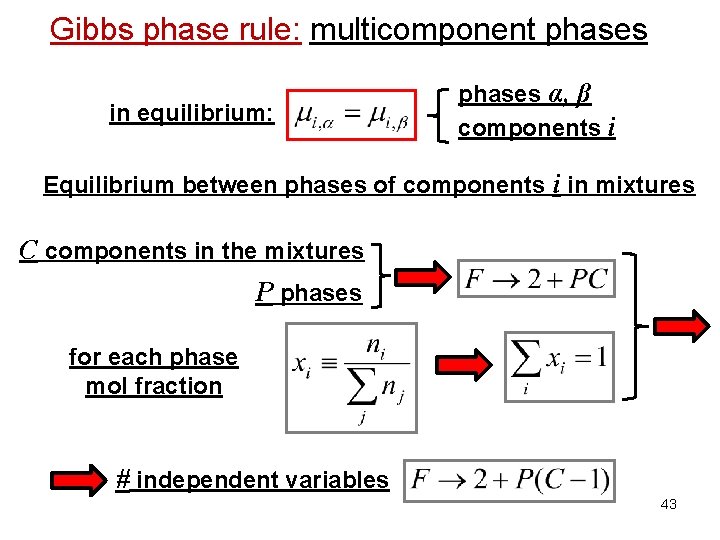
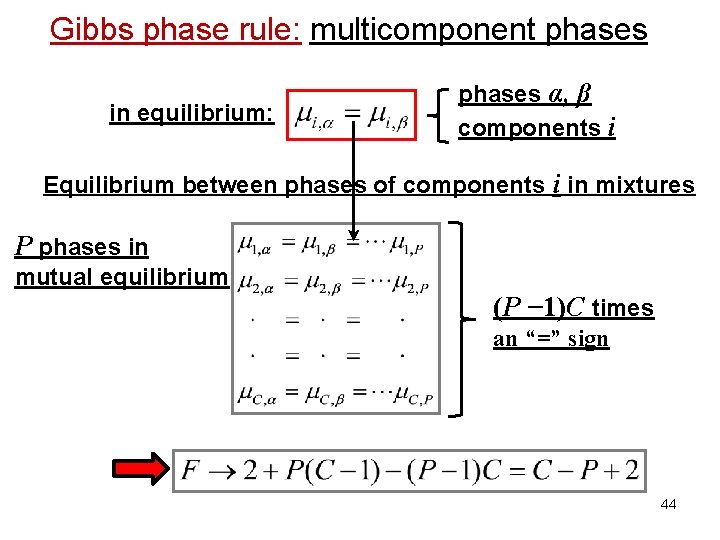
Gibbs phase rule: multicomponent phases Importance of the chemical potential: Equilibrium between phases in equilibrium: Equilibrium between phases of component i in mixtures phase α phase β in equilibrium: phases α, β components i 38

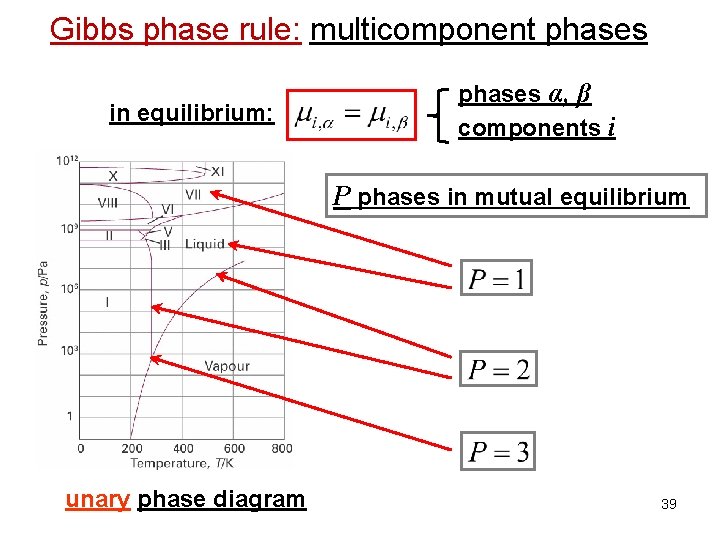
Gibbs phase rule: multicomponent phases in equilibrium: phases α, β components i P phases in mutual equilibrium unary phase diagram 39

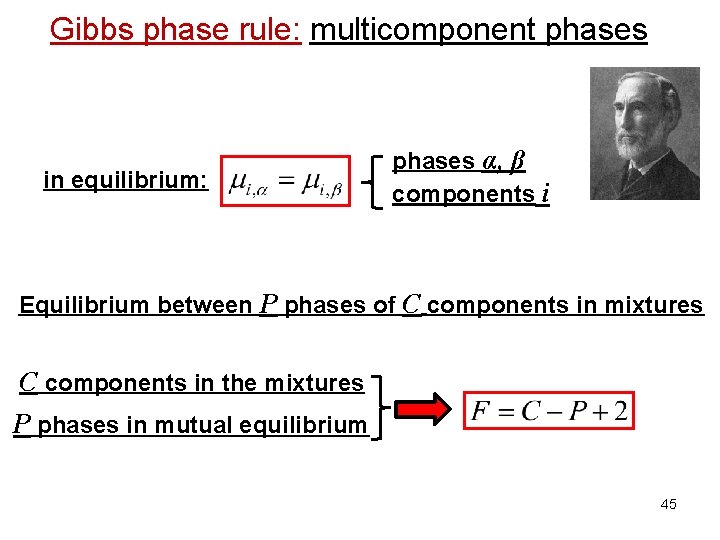
Gibbs phase rule: multicomponent phases α, β components i in equilibrium: Equilibrium between phases of components i in mixtures mole fraction C components in the mixtures # independent variables whole for each system phase 40

Gibbs phase rule: multicomponent phases α, β components i in equilibrium: Equilibrium between phases of components i in mixtures C components in the mixtures P phases in mutual equilibrium # independent variables whole for each system phase 41

Gibbs phase rule: multicomponent phases α, β components i in equilibrium: Equilibrium between phases of components i in mixtures # independent variables for each phase P phases 42

Gibbs phase rule: multicomponent phases in equilibrium: phases α, β components i Equilibrium between phases of components i in mixtures C components in the mixtures P phases for each phase mol fraction # independent variables 43

Gibbs phase rule: multicomponent phases in equilibrium: phases α, β components i Equilibrium between phases of components i in mixtures P phases in mutual equilibrium (P − 1)C times an “=” sign 44

Gibbs phase rule: multicomponent phases in equilibrium: phases α, β components i Equilibrium between P phases of C components in mixtures C components in the mixtures P phases in mutual equilibrium 45

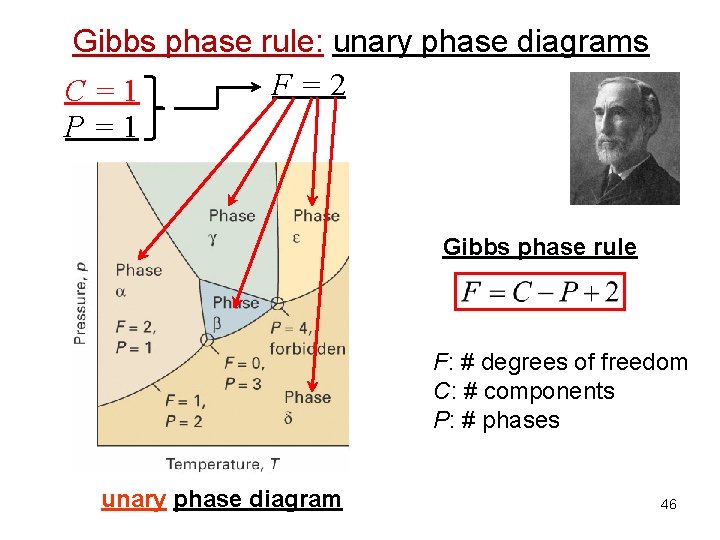
Gibbs phase rule: unary phase diagrams F=2 C=1 P=1 Gibbs phase rule F: # degrees of freedom C: # components P: # phases unary phase diagram 46

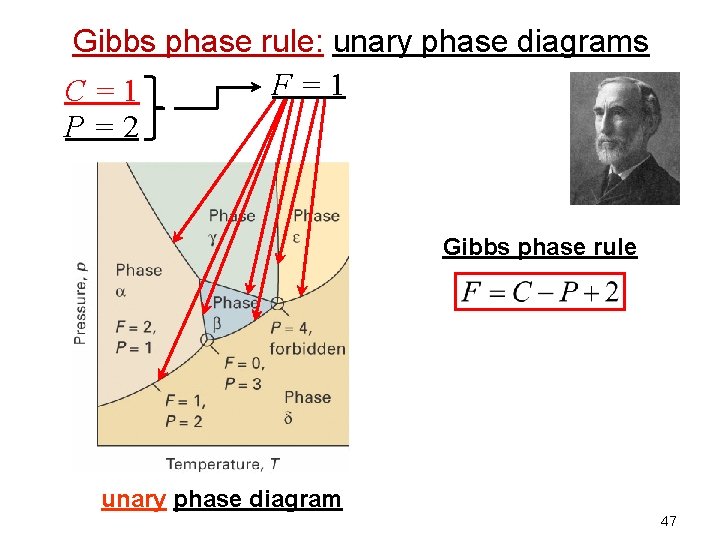
Gibbs phase rule: unary phase diagrams F=1 C=1 P=2 Gibbs phase rule unary phase diagram 47

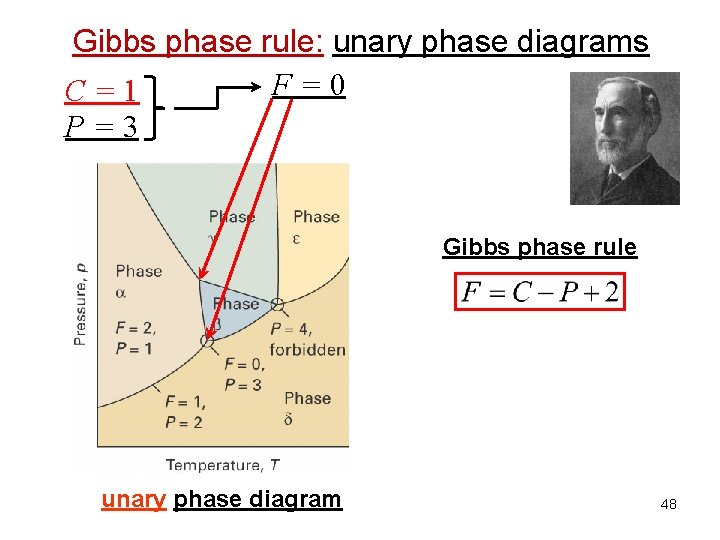
Gibbs phase rule: unary phase diagrams F=0 C=1 P=3 Gibbs phase rule unary phase diagram 48

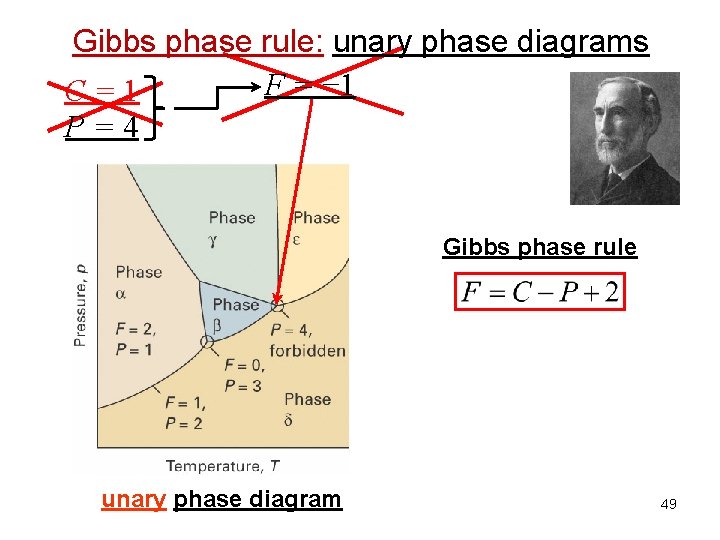
Gibbs phase rule: unary phase diagrams F = − 1 C=1 P=4 Gibbs phase rule unary phase diagram 49

50