Soils and Hydrology Lab 6 Cation Exchange Capacity

Soils and Hydrology - Lab 6 Cation Exchange Capacity (CEC) and Base Saturation (BS)

CEC and BS CEC (cation exchange capacity) is important for knowing the ability of a soil to store cations BS (base saturation) is important for knowing how many “good” cations are in a soil Method • Extract exchangeable cations • Measure base cations by AA spectroscopy • Measure acid cations by titration • Calculate CEC and BS • Convert from meq/100 g to lbs/ac

PROCEDURE Add 3 g soil to 50 m. L centrifuge tube Add 35 m. L Na. Cl solution Shake for 20 min Label your tube Centrifuge Filter into Erlenmeyer flask
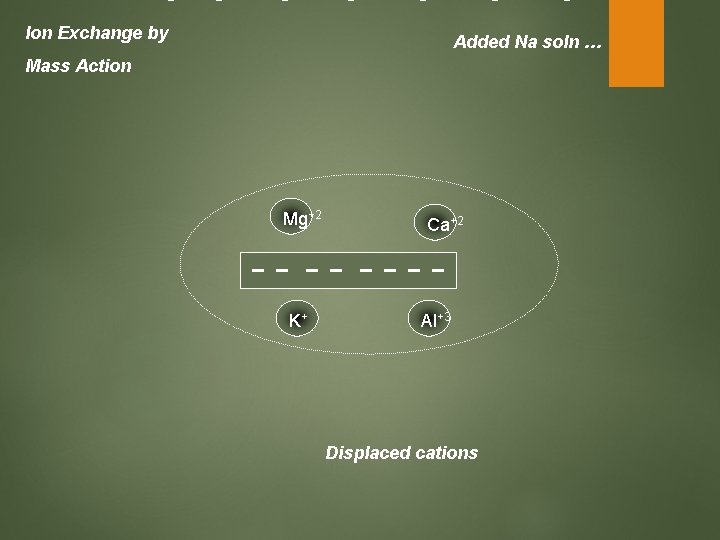
Ion Exchange by Added Na soln … Mass Action Mg+2 K+ Ca+2 Al+3 Displaced cations

Exchangeable Cations Once the centrifuge tube has been removed from the centrifuge, filter your sample into an Erlenmeyer flask Fill small vial with filtrate Transfer remaining filtrate to a graduated cylinder, record volume, and then return filtrate to Erlenmeyer flask youtube. com/watch? v=Hm. Eyym. GXOf. I

CEC and BS procedure 35 m. L 1 M Na. Cl 3 g Soil 40 m. L Centrifuge Tube C E N T R I F U G E Funnel w Filter Paper Small Vial Base Cations by AA Filtrate Small Flask Exchangeable ions by titration Shake 20 Minutes 50 m. L Graduated Cylinder

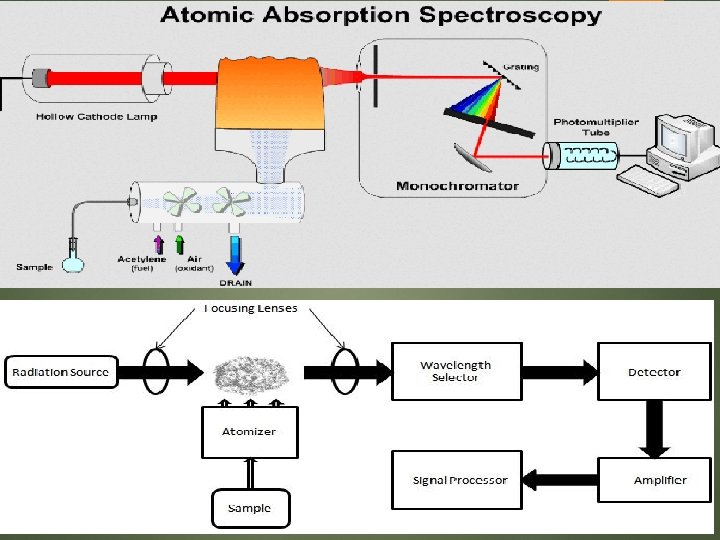
Atomic Adsorption Spectrometer


Titration of Acidic Cations with Burette

Exchangeable acids by titration Record volume of filtrate, VA Read initial value of Na. OH (scrimmage line) Add stir bar and a packet of phenolphthalein indicator to Erlenmeyer flask Turn on stirrer to slow speed. Slowly add Na. OH (drop by drop), NB Stop when solution stays pink for 20 -30 s Read final value of Na. OH (position at end of down) Record volume base used, VB (yards advanced!) Remember: amount of added base equals original amount of acid, so that NB x V B = N A x V A

Titration Calculations: NA = N B x V B / V A NA is acid concentration (meq/m. L) NB is base concentration (0. 0044 meq/m. L) VB is volume of base (m. L) from burette VA is volume of acid (m. L) from graduated cylinder

Solution Concentration vs Soil Concentration Our cations were extracted from 3 g of soil These cations are now in 35 m. L of solution We have to adjust our solution concentration back to the original soil concentration meq acid m. L soln meq Ca L soln x x 35 m. L soln = meq acid 3 g soil 0. 035 L soln = meq Ca 3 g soil We then multiply by 100 to get meq/100 g
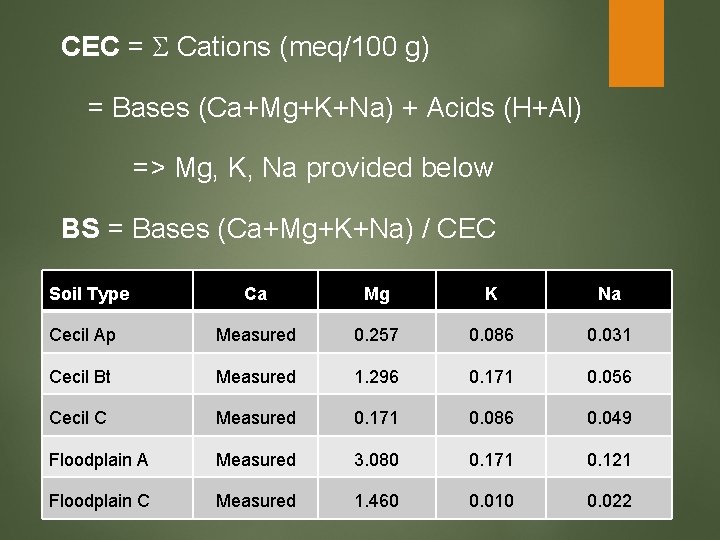
CEC = Cations (meq/100 g) = Bases (Ca+Mg+K+Na) + Acids (H+Al) => Mg, K, Na provided below BS = Bases (Ca+Mg+K+Na) / CEC Soil Type Ca Mg K Na Cecil Ap Measured 0. 257 0. 086 0. 031 Cecil Bt Measured 1. 296 0. 171 0. 056 Cecil C Measured 0. 171 0. 086 0. 049 Floodplain A Measured 3. 080 0. 171 0. 121 Floodplain C Measured 1. 460 0. 010 0. 022

Cations Monovalent (H, K, Na): 1 mole = 1 equivalent Divalent (Ca, Mg): 1 mole = 2 equivalents Trivalent (Al, Fe): 1 mole = 3 equivalents And remember… 1 mg/kg = 1 ppm = 2 lbs/ac
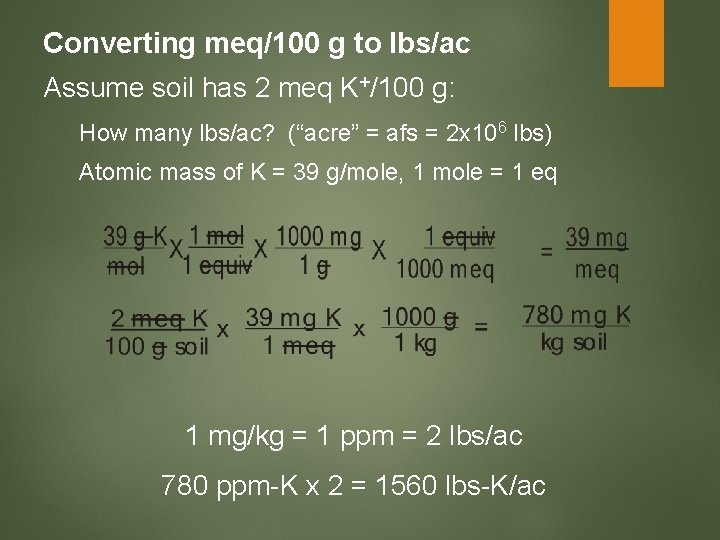
Converting meq/100 g to lbs/ac Assume soil has 2 meq K+/100 g: How many lbs/ac? (“acre” = afs = 2 x 106 lbs) Atomic mass of K = 39 g/mole, 1 mole = 1 eq 1 mg/kg = 1 ppm = 2 lbs/ac 780 ppm-K x 2 = 1560 lbs-K/ac

Show All Steps of Calculations Including Units Please Write Legibly Hand In Lab Report Next Week
- Slides: 17