Soil Microbiology ENVIRONMENTAL MICROBIOLOGY WHAT IS SOIL Soils

Soil Microbiology ENVIRONMENTAL MICROBIOLOGY
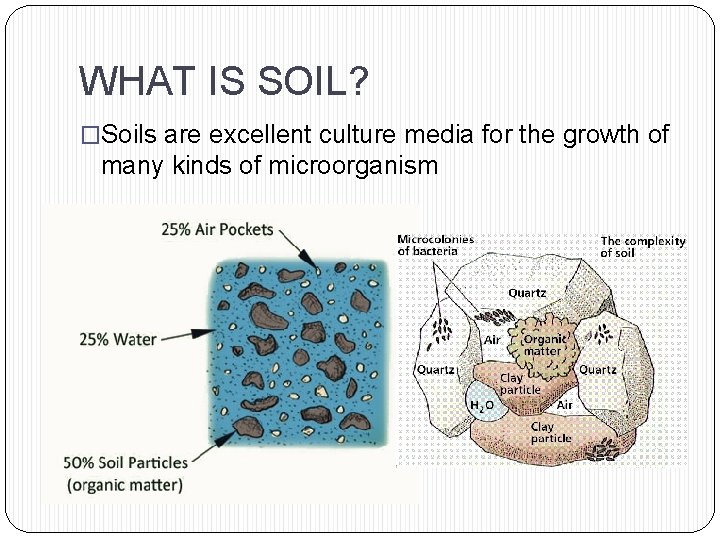
WHAT IS SOIL? �Soils are excellent culture media for the growth of many kinds of microorganism

Who is at home in the soil? Soils are excellent culture media for the growth of many kinds of microorganism
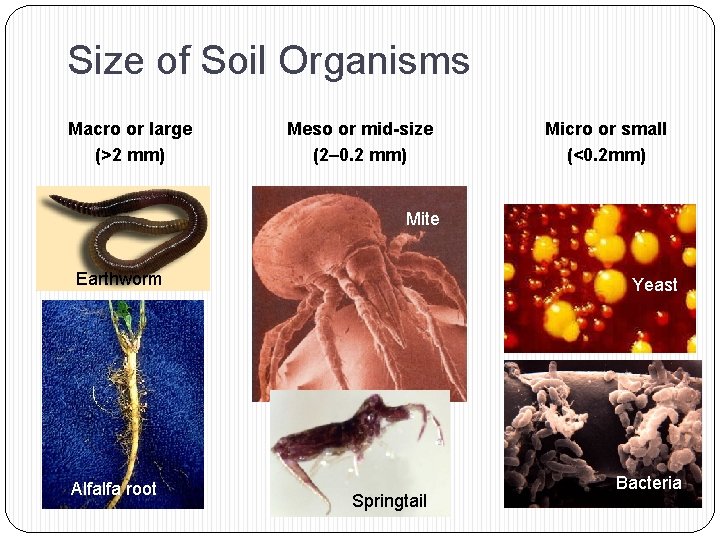
Size of Soil Organisms Macro or large (>2 mm) Meso or mid-size (2– 0. 2 mm) Micro or small (<0. 2 mm) Mite Earthworm Alfalfa root Yeast Springtail Bacteria
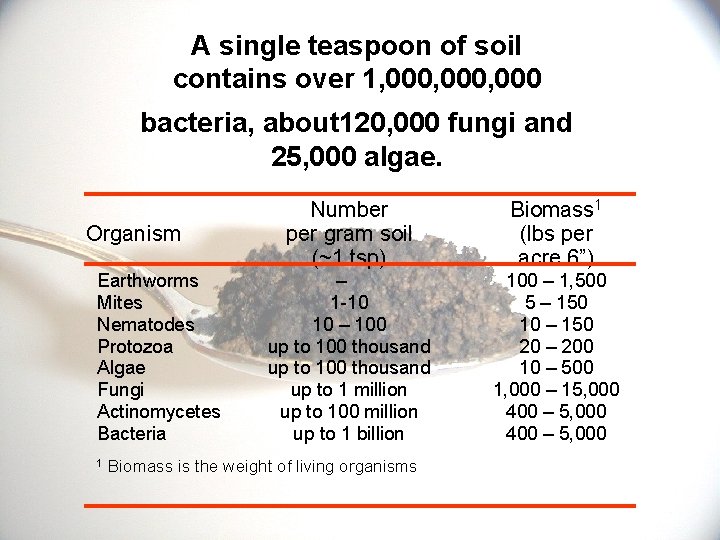
A single teaspoon of soil contains over 1, 000, 000 bacteria, about 120, 000 fungi and 25, 000 algae. Organism Earthworms Mites Nematodes Protozoa Algae Fungi Actinomycetes Bacteria 1 Number per gram soil (~1 tsp) Biomass 1 (lbs per acre 6”) – 1 -10 10 – 100 up to 100 thousand up to 1 million up to 100 million up to 1 billion 100 – 1, 500 5 – 150 10 – 150 20 – 200 10 – 500 1, 000 – 15, 000 400 – 5, 000 Biomass is the weight of living organisms
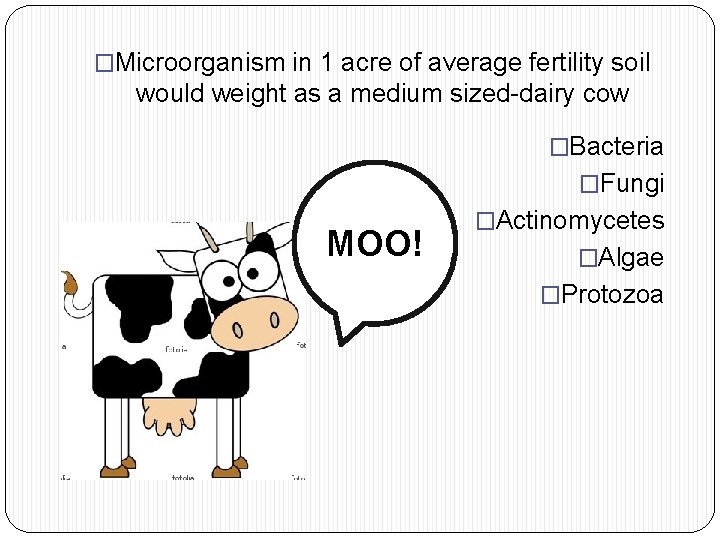
�Microorganism in 1 acre of average fertility soil would weight as a medium sized-dairy cow �Bacteria �Fungi MOO! �Actinomycetes �Algae �Protozoa

The world of microorganisms in soil
Bacteria � Bacterial population of the soil exceeds other groups of microorganisms in number and variety. � Plate count yields 1 -10% of the total count (as compared to direct microscopic counts). � Dominant genera : Arthrobacter, Pseudomonas, Agrobacterium, Flavobacterium and Bacillus. � Animal pathogens : Clostridium, Bacillus, Coxiella and Streptococcus � Plant pathogens : Agrobacterium, Erwinia, Corynebacterium, Pseudomonas and Xanthomonas. � Different physiological groups are also present.

Where are the microorganisms located in the soil? � Usually the top 2 -3 cms. � Commonly found close to root surfaces, in dead roots, on soil particles or amongst aggregates of soil particles. � Soils that are clayey have many bacteria because these soils have lot of small pores. � Sandy soil is less suitable habitat.

Fertile Soil Clay Soil Infertile Soil Sandy Soil
Soil factors that affect microorganism growth Spherical Bacteria �Organic matter �Aeration (oxygen) �Moisture and temperature �Soil fertility and p. H

Rod-Shaped Bacteria
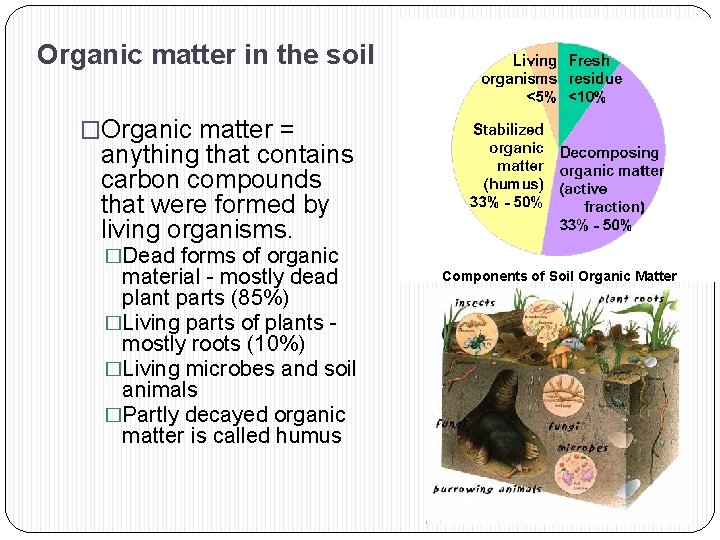
Organic matter in the soil �Organic matter = anything that contains carbon compounds that were formed by living organisms. �Dead forms of organic material - mostly dead plant parts (85%) �Living parts of plants mostly roots (10%) �Living microbes and soil animals �Partly decayed organic matter is called humus Components of Soil Organic Matter
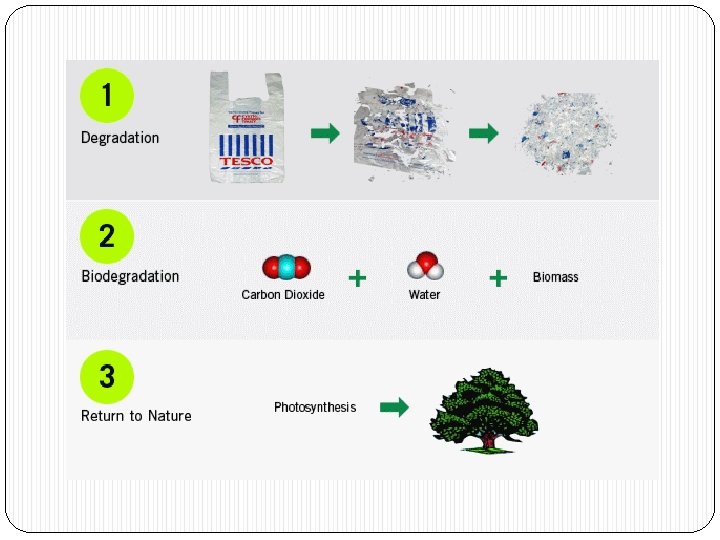
Microbial degradation or organic matter �Involves complex processes. Chemical alteration of organic matter, physical fragmentation and release of mineral nutrients. �The dead organic matter is colonized by microbes and degraded with help of microbial enzymes. �Macromolecules are broken down into simpler units and further degraded into constituent elements.
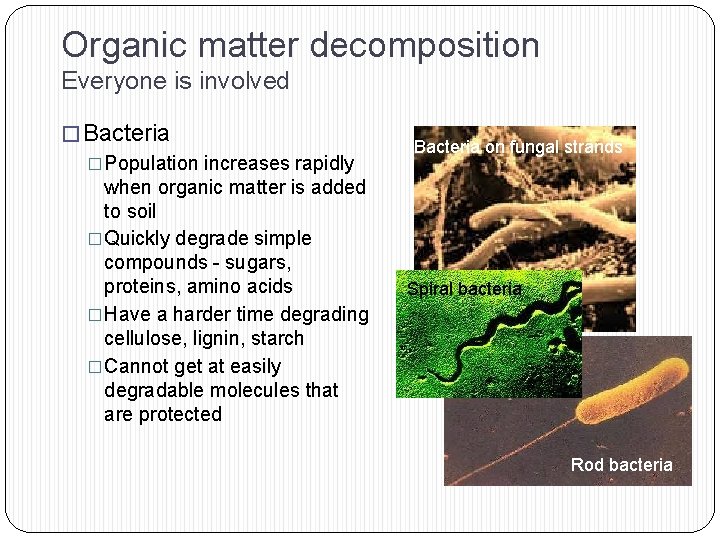
Organic matter decomposition Everyone is involved � Bacteria �Population increases rapidly when organic matter is added to soil �Quickly degrade simple compounds - sugars, proteins, amino acids �Have a harder time degrading cellulose, lignin, starch �Cannot get at easily degradable molecules that are protected Bacteria on fungal strands Spiral bacteria Rod bacteria
Effect of soil temperature 4 Microorganisms have been found growing in virtually all environments where there is liquid water, regardless of its temperature. 4 Microorganisms are found growing at the sub zero temperatures of Antarctic soil to temperatures as high as 115 o. C in deep sea hydrothermal vents.
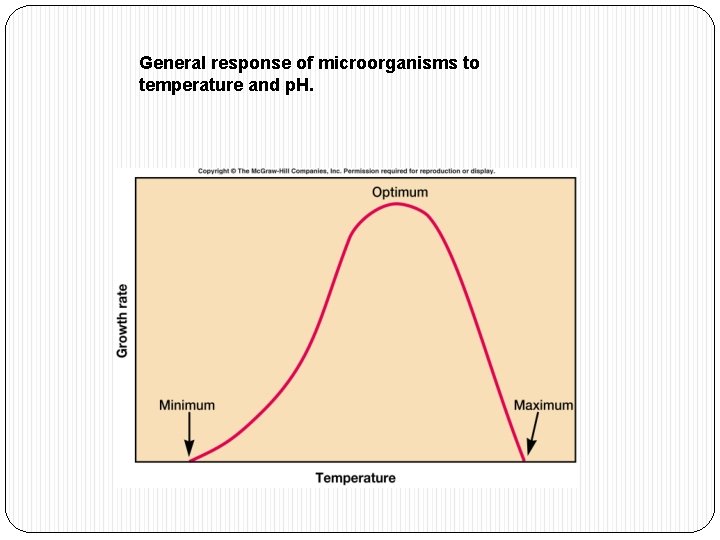
General response of microorganisms to temperature and p. H.
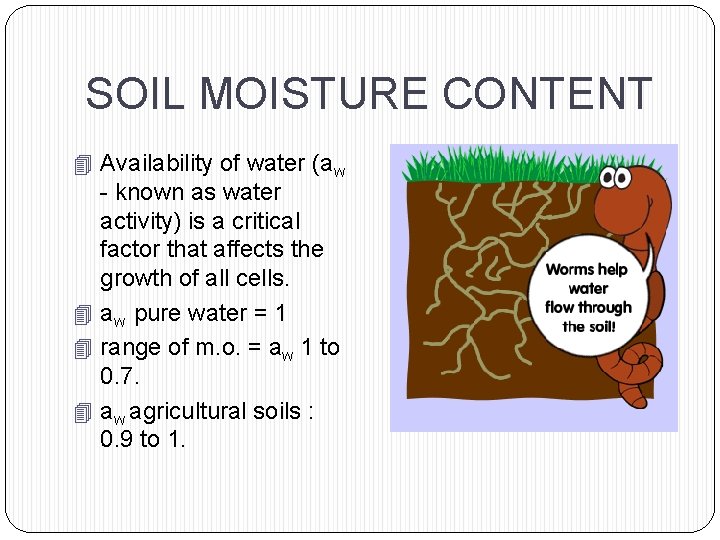
SOIL MOISTURE CONTENT 4 Availability of water (aw - known as water activity) is a critical factor that affects the growth of all cells. 4 aw pure water = 1 4 range of m. o. = aw 1 to 0. 7. 4 aw agricultural soils : 0. 9 to 1.
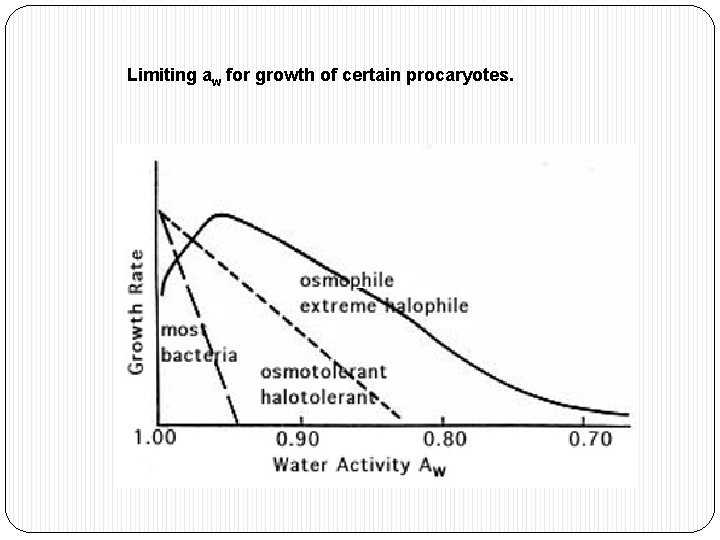
Limiting aw for growth of certain procaryotes.
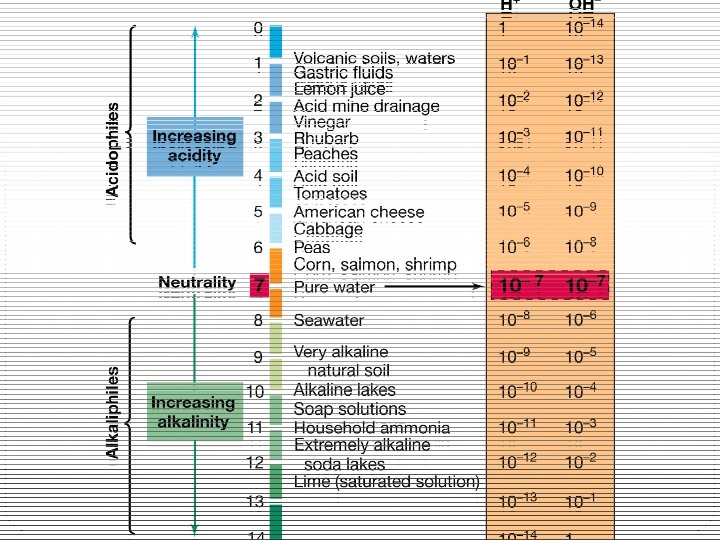
Soil p. H 4 The p. H or hydrogen ion concentration of natural environments varies from about 0. 5 in the most acidic soils to about 10. 5 in the most alkaline lakes. 4 Most free living prokaryotes can grow over a range of p. H. 4 Depending on p. H preferences microorganisms are classified into – acidophiles, neutrophiles and alkaliphiles.

Sources of N �Lightning �Inorganic fertilizers �Nitrogen Fixation �Animal Residues �Crop residues �Organic fertilizers
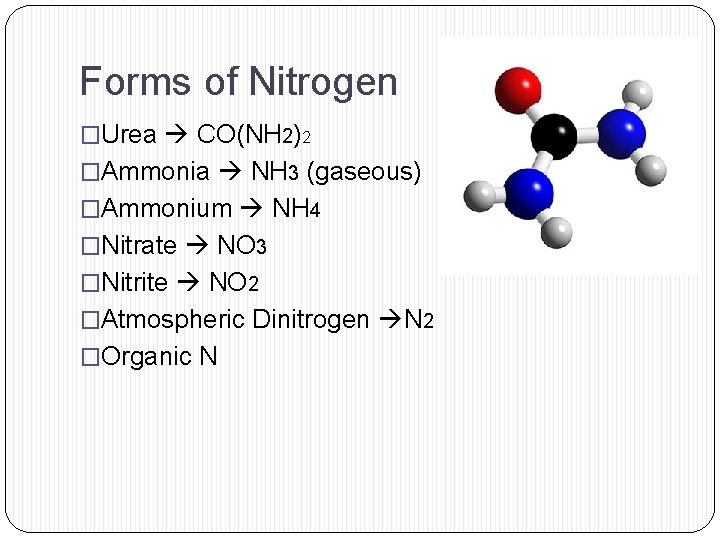
Forms of Nitrogen �Urea CO(NH 2)2 �Ammonia NH 3 (gaseous) �Ammonium NH 4 �Nitrate NO 3 �Nitrite NO 2 �Atmospheric Dinitrogen N 2 �Organic N
Roles of Nitrogen �Plants and bacteria use nitrogen in the form of NH 4+ or NO 3�It serves as an electron acceptor in anaerobic environment �Nitrogen is often the most limiting nutrient in soil and water. Nitrogen is a key element for � amino acids � nucleic acids (purine, pyrimidine) �cell wall components of bacteria (NAM).
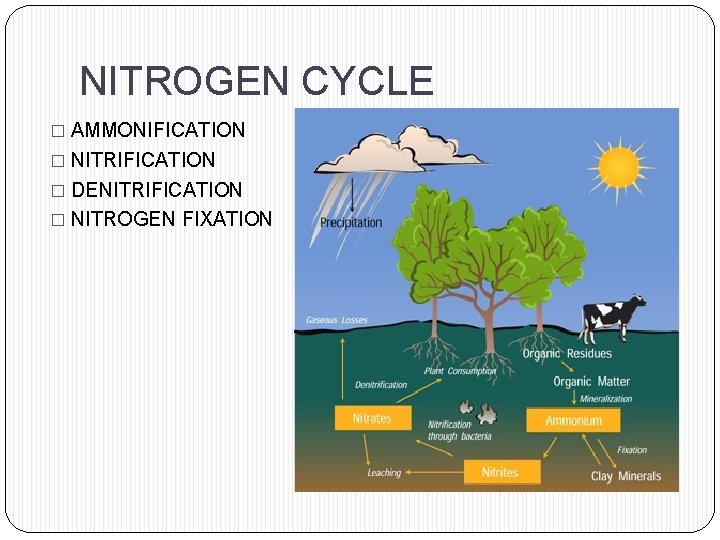
NITROGEN CYCLE � AMMONIFICATION � NITRIFICATION � DENITRIFICATION � NITROGEN FIXATION
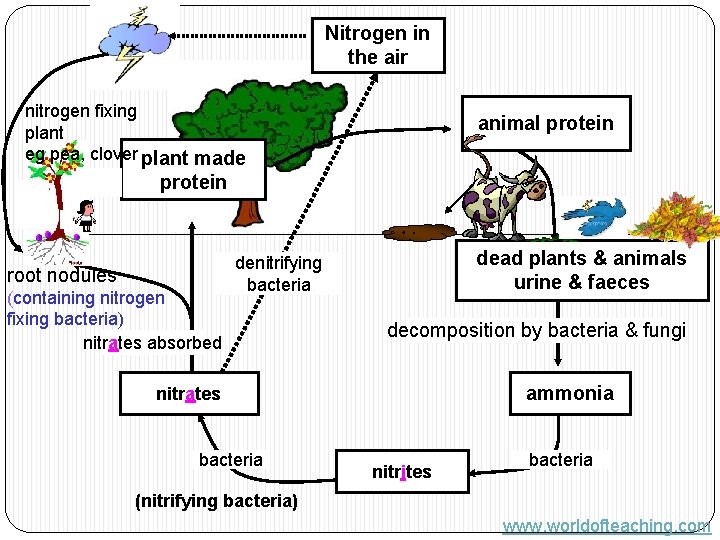
Nitrogen in the air nitrogen fixing plant eg pea, clover plant made animal protein root nodules (containing nitrogen fixing bacteria) nitrates absorbed dead plants & animals urine & faeces denitrifying bacteria decomposition by bacteria & fungi ammonia nitrates bacteria nitrites bacteria (nitrifying bacteria) www. worldofteaching. com
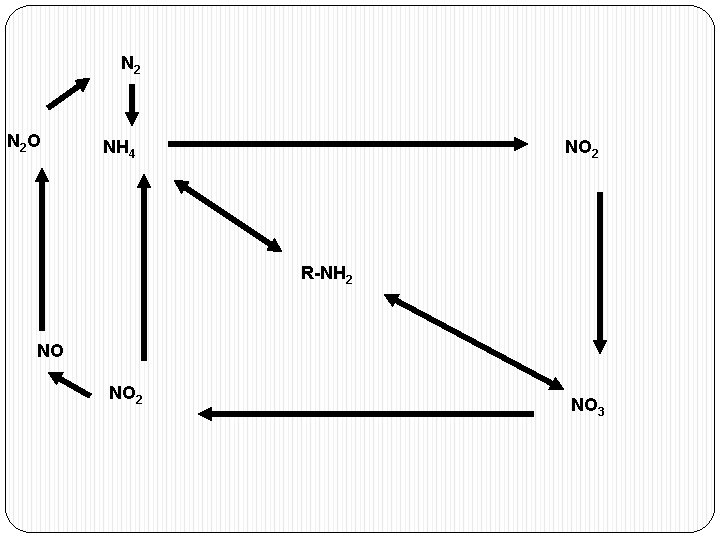
N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
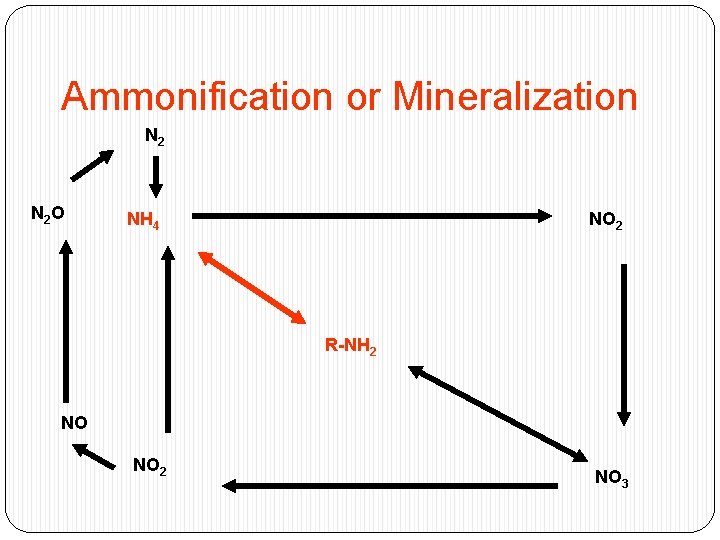
Ammonification or Mineralization N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
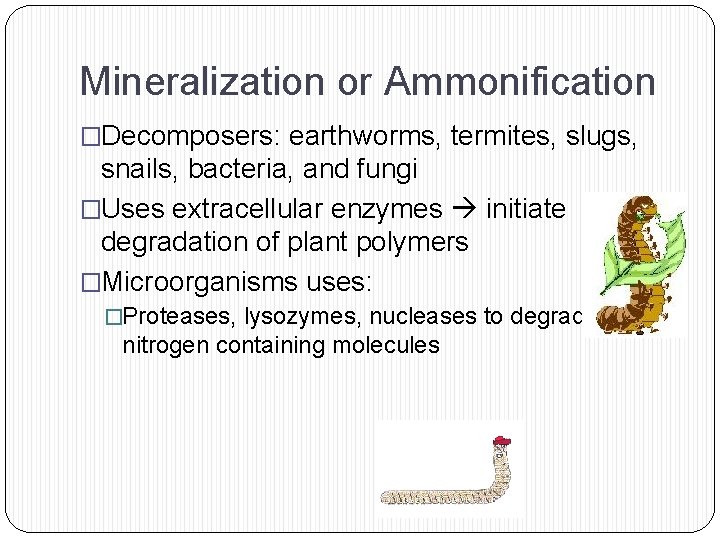
Mineralization or Ammonification �Decomposers: earthworms, termites, slugs, snails, bacteria, and fungi �Uses extracellular enzymes initiate degradation of plant polymers �Microorganisms uses: �Proteases, lysozymes, nucleases to degrade nitrogen containing molecules
�Plants die or bacterial cells lyse release of organic nitrogen �Organic nitrogen is converted to inorganic nitrogen (NH 3) �When p. H<7. 5, converted rapidly to NH 4 �Example: Urea NH 3 + 2 CO 2
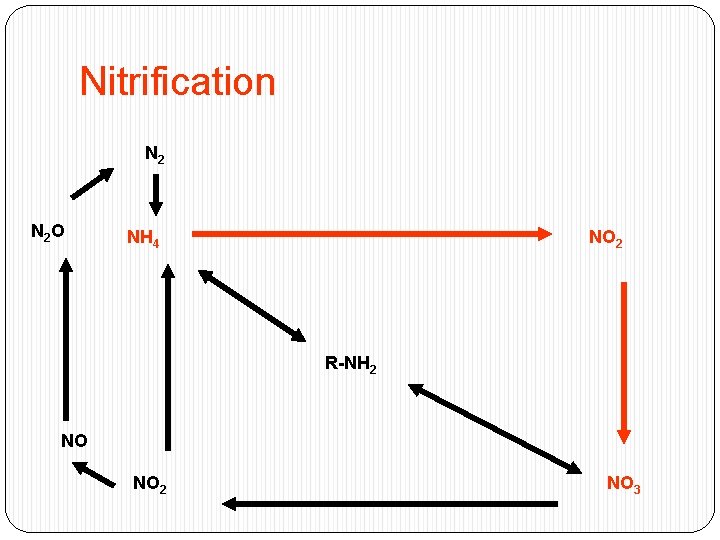
Nitrification N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
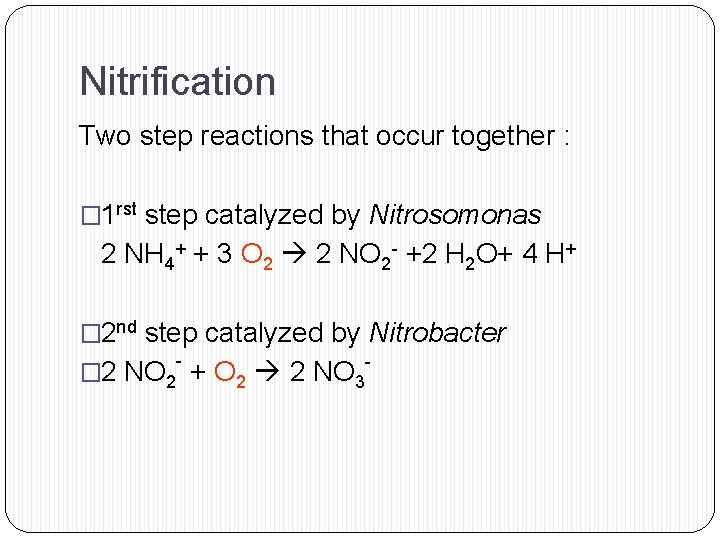
Nitrification Two step reactions that occur together : � 1 rst step catalyzed by Nitrosomonas 2 NH 4+ + 3 O 2 2 NO 2 - +2 H 2 O+ 4 H+ � 2 nd step catalyzed by Nitrobacter - � 2 NO 2 + O 2 2 NO 3 -
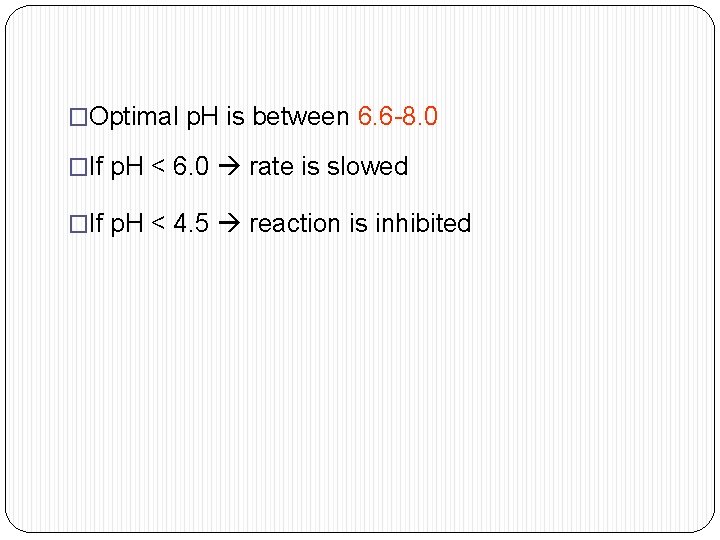
�Optimal p. H is between 6. 6 -8. 0 �If p. H < 6. 0 rate is slowed �If p. H < 4. 5 reaction is inhibited
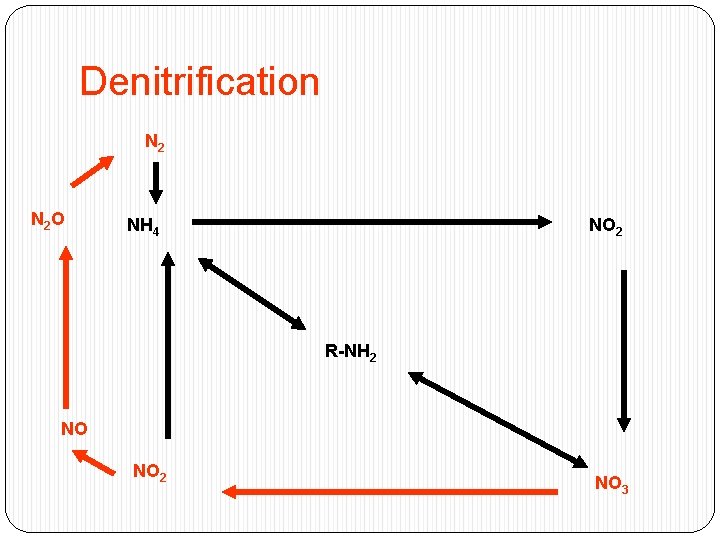
Denitrification N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
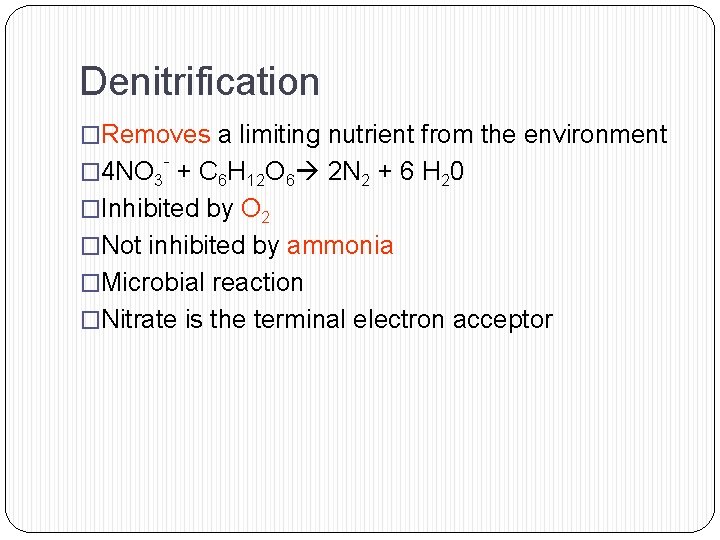
Denitrification �Removes a limiting nutrient from the environment - � 4 NO 3 + C 6 H 12 O 6 2 N 2 + 6 H 20 �Inhibited by O 2 �Not inhibited by ammonia �Microbial reaction �Nitrate is the terminal electron acceptor
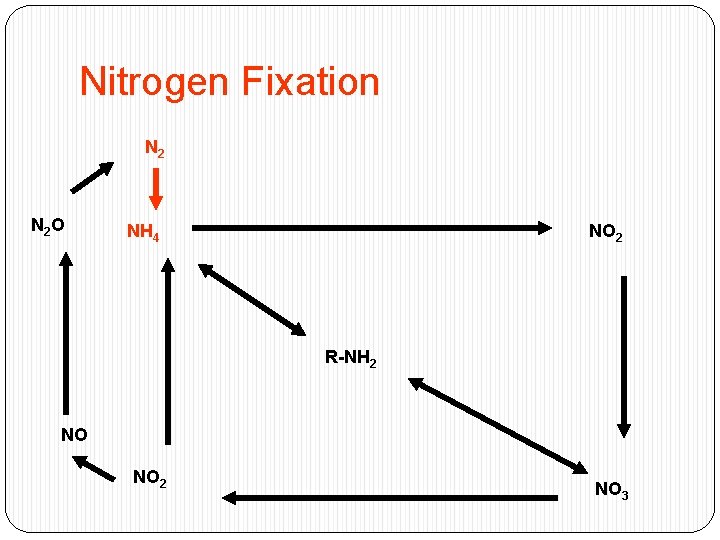
Nitrogen Fixation N 2 O NH 4 NO 2 R-NH 2 NO NO 2 NO 3
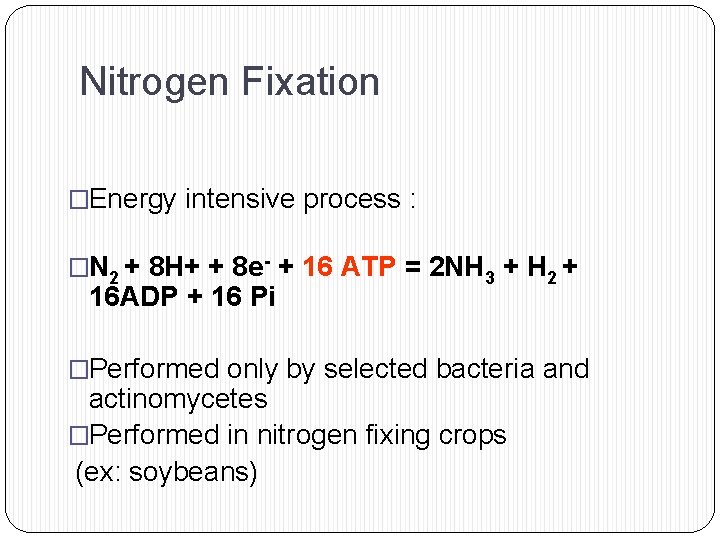
Nitrogen Fixation �Energy intensive process : �N 2 + 8 H+ + 8 e- + 16 ATP = 2 NH 3 + H 2 + 16 ADP + 16 Pi �Performed only by selected bacteria and actinomycetes �Performed in nitrogen fixing crops (ex: soybeans)
Microorganisms fixing �Azobacter �Require the enzyme �Beijerinckia nitrogenase �Inhibited by oxygen �Inhibited by ammonia (end product) �Azospirillum �Clostridium �Cyanobacteria

The nitrogen fixing bacteria are found inlumps on the roots called root nodules. The bacteria and the plant have a symbiotic relationship: the bacteria benefits by having food and shelter from the plant and the plant benefits by having nitrates produced by the bacteria. Roots of a legume plant (peas, beans and clover). www. worldofteaching. com
- Slides: 41