Soil formation in dry climates Calcification forms calcic





































- Slides: 50

Soil formation in dry climates

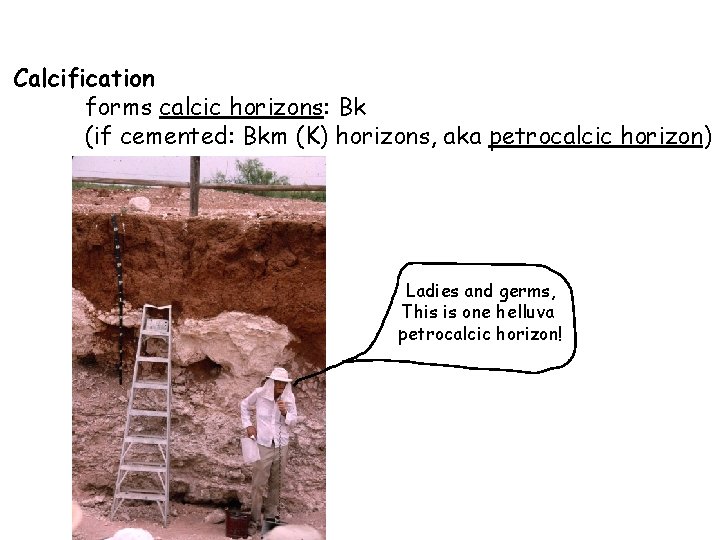
Calcification forms calcic horizons: Bk (if cemented: Bkm (K) horizons, aka petrocalcic horizon) Ladies and germs, This is one helluva petrocalcic horizon!

Two factors to always consider: 1. Dust is everywhere

Dust devil trails

Namibia

Approaching alkalai dust storm, off a playa to the left

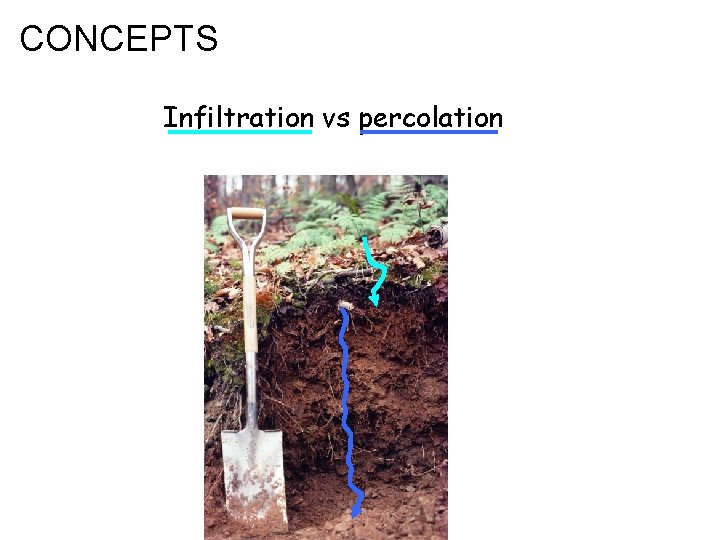
CONCEPTS Infiltration vs percolation

CONCEPTS Potential evapotranspiration – max (potential) water that can be evaporated and transpired from an area PET

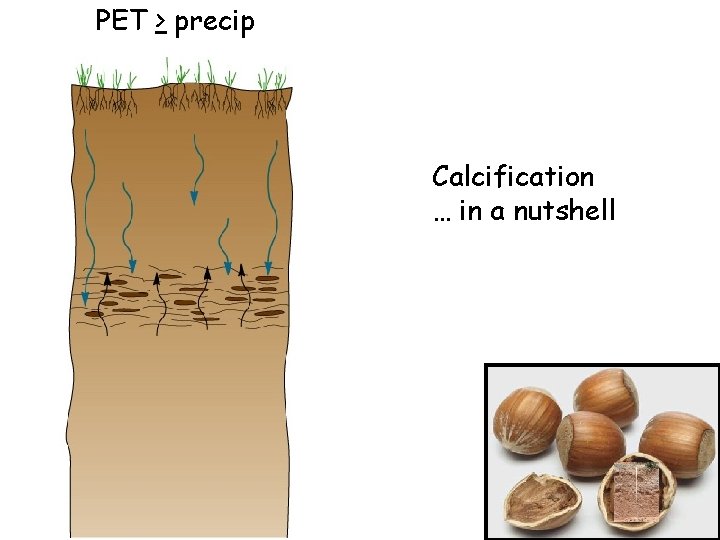
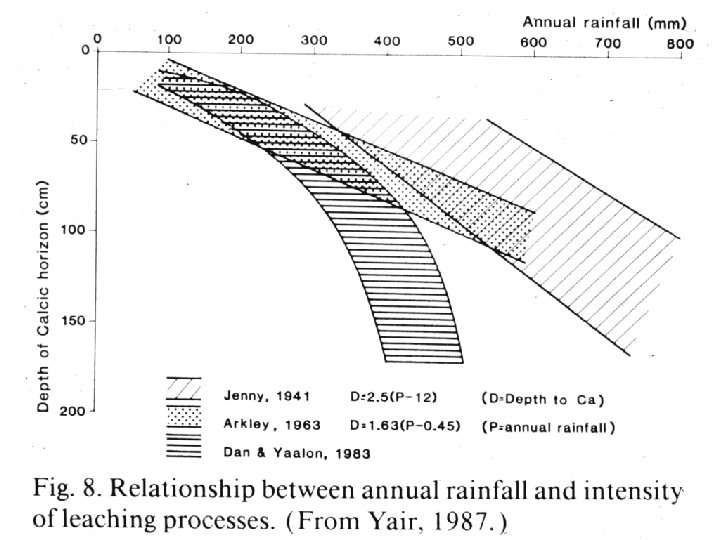
Two factors to always consider: 1. Dust is everywhere 2. Where PET > P, precip infiltrates but rarely does it percolate THROUGH the profile Instead, the soil gets wetted to a given depth, but the moisture then wicks back up to the surface Any soluble compounds in the wetted soil precipitate in the profile

PET > precip Calcification … in a nutshell

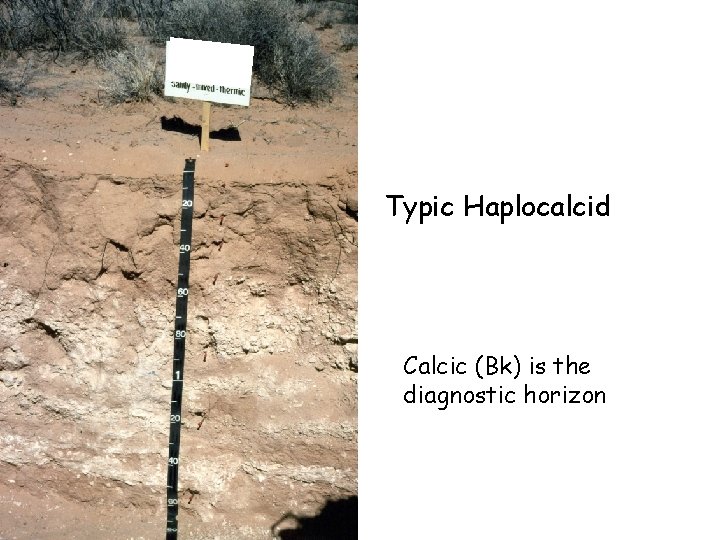
Typic Haplocalcid Calcic (Bk) is the diagnostic horizon

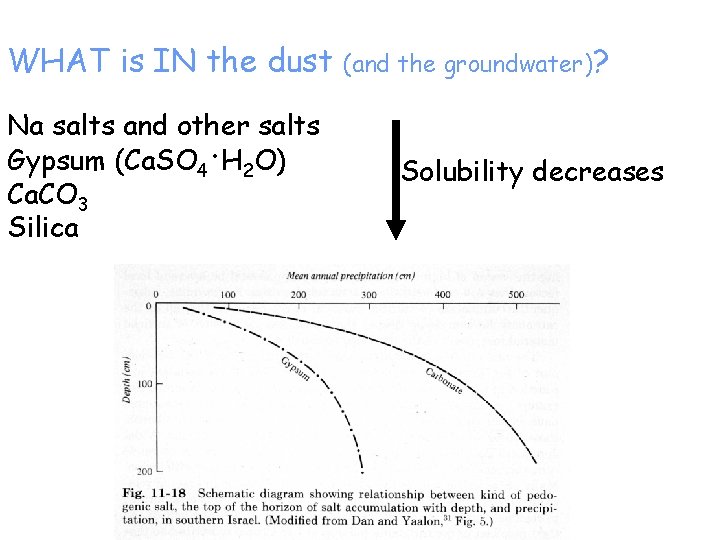
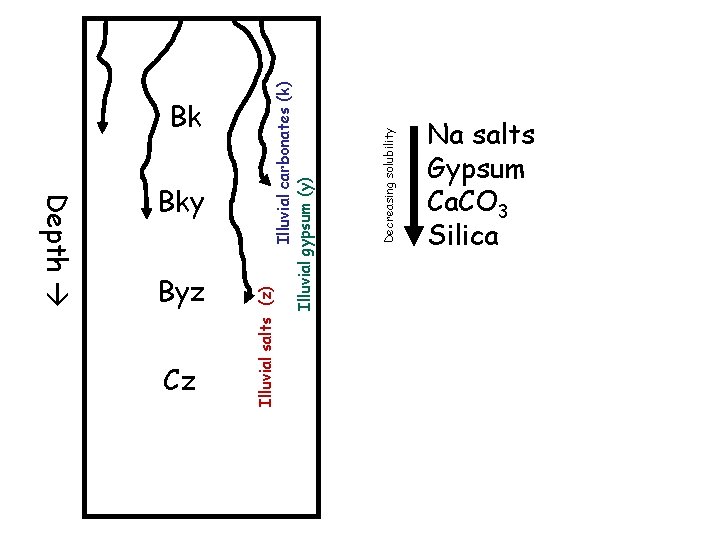
In dry climates, B horizons accumulate soluble materials, translocated in percolating water: By – gypsum – gypsic horizon Bk – carbonates – calcic horizon Bz – soluble salts – salic horizon Bn – Na salts – natric horizon Cemented versions Bq – silica Bkm – caliche, calcrete, petrocalcic horizon Bqm – duripan, silcrete Bym – gypcrete, petrogypsic horizon

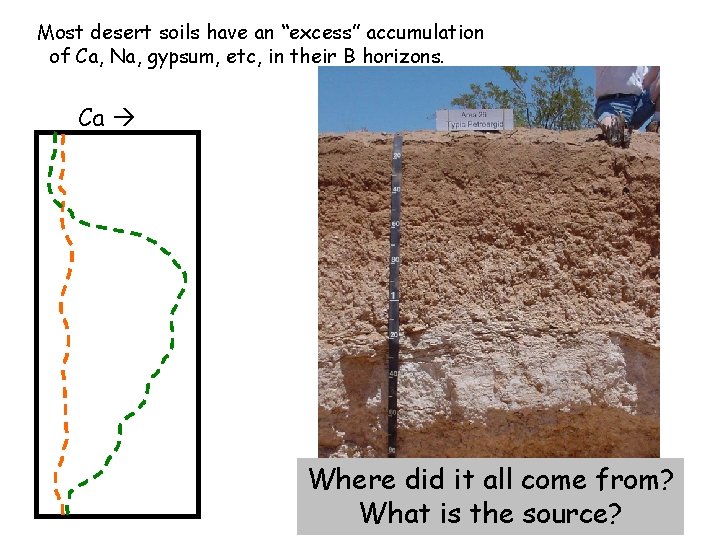
Most desert soils have an “excess” accumulation of Ca, Na, gypsum, etc, in their B horizons. Ca Where did it all come from? What is the source?

WHAT is IN the dust Na salts and other salts Gypsum (Ca. SO 4. H 2 O) Ca. CO 3 Silica (and the groundwater)? Solubility decreases

Depth Byz Cz Decreasing solubility Illuvial carbonates (k) Illuvial gypsum (y) Bky Illuvial salts (z) Bk Na salts Gypsum Ca. CO 3 Silica

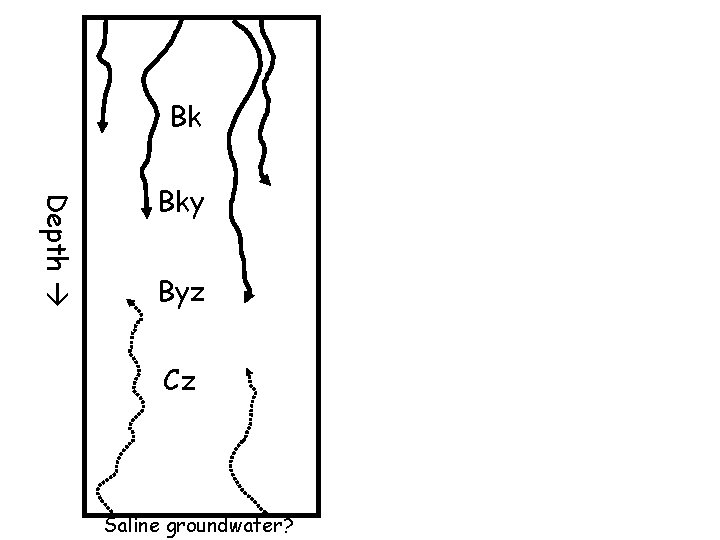
Bk Depth Bky Byz Cz Saline groundwater?


Typic Haplosalid



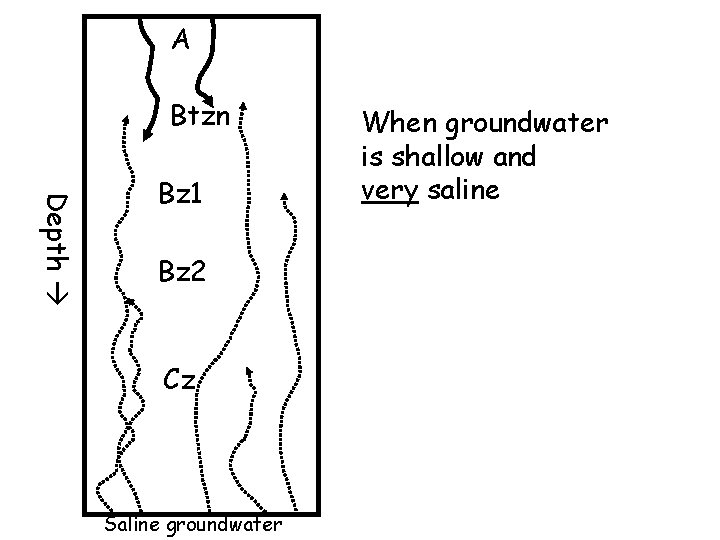
A Btzn Depth Bz 1 Bz 2 Cz Saline groundwater When groundwater is shallow and very saline

Sooooooo, …. it all depends on 1. What is available (from dust, groundwater, etc) 2. Solubility Na salts Gypsum Ca. CO 3 Silica Solubility decreases

From here on in, our focus will be on carbonates

Carbonates are normally translocated from the surface to depth – the per descensum model

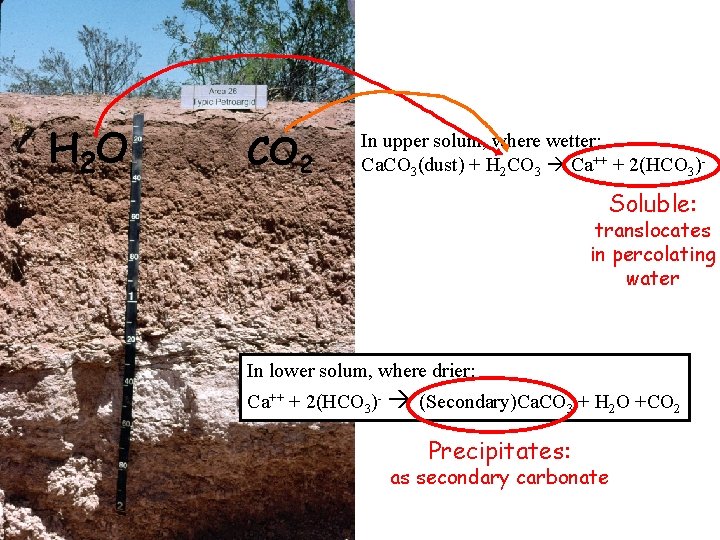
H 2 O CO 2 In upper solum, where wetter: Ca. CO 3(dust) + H 2 CO 3 Ca++ + 2(HCO 3)- Soluble: translocates in percolating water In lower solum, where drier: Ca++ + 2(HCO 3)- (Secondary)Ca. CO 3 + H 2 O +CO 2 Precipitates: as secondary carbonate

Thus, to get Ca. CO 3 precipitation: -dry conditions (stoppage of wetting fronts)*** -rise in ionic concentration of soil solution (cessation of percolation) -lowering of CO 2 in soil air --warmer temps at depth cold water is able to dissolve more Ca. CO 3 than warm water (important only regionally)




Stage 1


Carbonate filaments – Stage 1

Early Stage 2

Mid-Stage 2

Carbonates - Stage 2

Secondary carbonates under rocks – stages 1 and 2

Stage 3 – Bkm develops. Carbonates plug pores. Bkm becomes aquitard, then an aquiclude

Stage 3

Late Stage 3

Late Stage 3

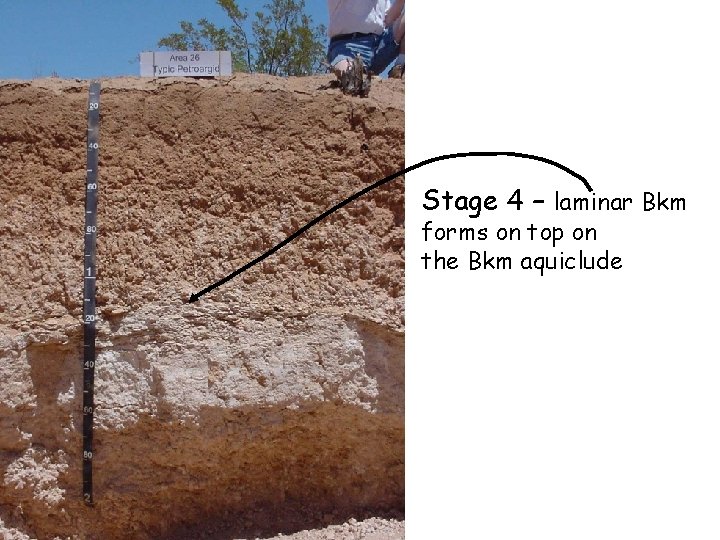
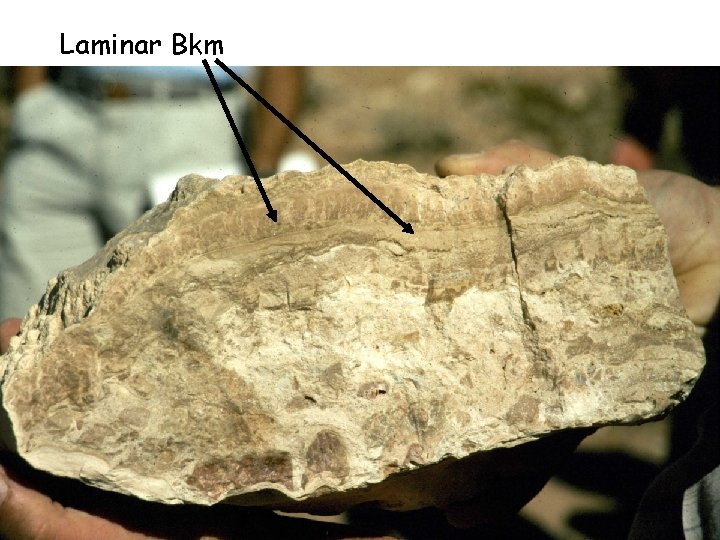
Stage 4 – laminar Bkm forms on top on the Bkm aquiclude

Solid Stage 4

Stage 4 – this is as deep as the backhoe could go!

Typic Petrocalcid

Laminar Bkm


Stages 5 -6 – Bkm begins to break up


Pisoliths
