Soil Flushing Soil flushing is an in situ




























- Slides: 44

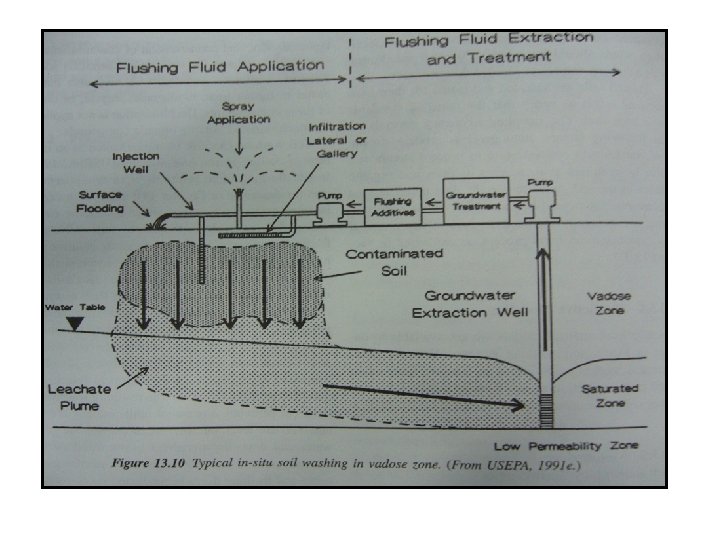
Soil Flushing • Soil flushing is an in situ technology for the removal of sorbed-phase contaminants from the vadose soil zone. It also can be applied to the treatment of contaminated soil located in the saturated zone. • The process accomplished by passing the flushing solvent using injection well or infiltration system into the areas of contamination to enhance contaminant solubility and transport the contaminant to aqueous phase. • After application, the flushing fluid percolate downward through the contaminated soil. Contaminants are mobilized through dissolution, or by chemical reaction with the flushing fluid additive. • The resulting leachate continue to percolate downward until reaching the water table. At this level, leachate mix with groundwater and flow downgradient to the withdrawal point. The mobilized contaminants then collected and put through a treatment process which remove/treat the contaminants. • Treatment of the collected groundwater is required to remove the dissolved contaminants and solvents. The treated groundwater can be reinjected and used to mobilized the remaining contamination. If the collected groundwater can not be used, other disposal methods will be required and the remediation cost likely to be increased.


• Soil flushing methods remove contaminants by dissolving the sorbed and vapor phase or by mobilizing contaminants existing as free products in the soil pore or absorbed to the soils. • The former process are controlled by the solubility of the contaminants and Henry laws constant, while the latter are controlled by the viscosity of the contaminants. • The treatment solution for flushing a contaminants from soil can be delivered via gravity (flooding, spraying), surface seepage or forced system (injecting pipe). • Flushing or mobilizing wastes can serve 2 purposes: to promote the recovery of waste from the subsurface for treatment on the surface or to solubilize adsorbed compounds in order to enhance the rate of other in situ treatment technique such as biodegradation.

Flushing Solution/Solvent • Selection of an aqueous extracting agent is based upon safety to human and environment. • The specific contaminants in the soil at any particular site determine the type of flushing solution needed in the treatment process. • The ‘solvent’ is a chemical addition that solubilizes, emulsifies or chemically modifies the contaminants. • Soil flushing has been used for organic and inorganic contaminants. Surfactant is used for organic contaminants and chelating agent and/or acid for heavy metal contaminants.

• The solution used for in situ flushing may consist of one or more of the following: - plain water - surfactant/cosolvent - acid or base - oxidants/reductants - chelants • The flushing solution serve to increase the mobility and solubility of the contaminants

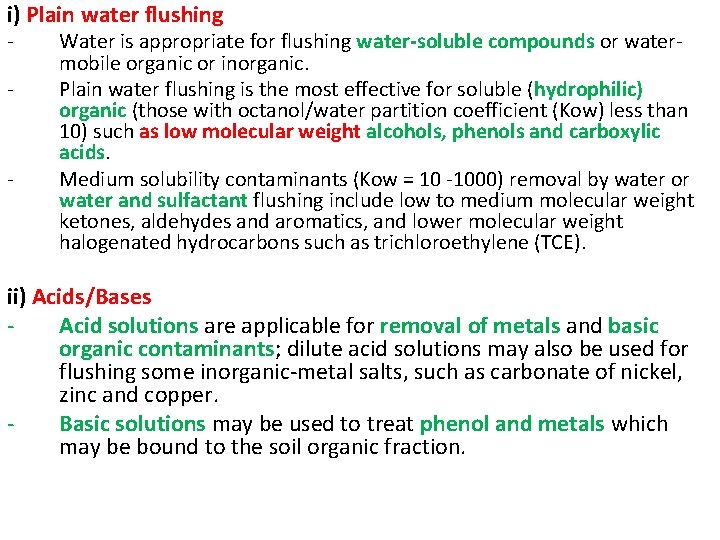
i) Plain water flushing - - Water is appropriate for flushing water-soluble compounds or watermobile organic or inorganic. Plain water flushing is the most effective for soluble (hydrophilic) organic (those with octanol/water partition coefficient (Kow) less than 10) such as low molecular weight alcohols, phenols and carboxylic acids. Medium solubility contaminants (Kow = 10 -1000) removal by water or water and sulfactant flushing include low to medium molecular weight ketones, aldehydes and aromatics, and lower molecular weight halogenated hydrocarbons such as trichloroethylene (TCE). ii) Acids/Bases Acid solutions are applicable for removal of metals and basic organic contaminants; dilute acid solutions may also be used for flushing some inorganic-metal salts, such as carbonate of nickel, zinc and copper. Basic solutions may be used to treat phenol and metals which may be bound to the soil organic fraction.

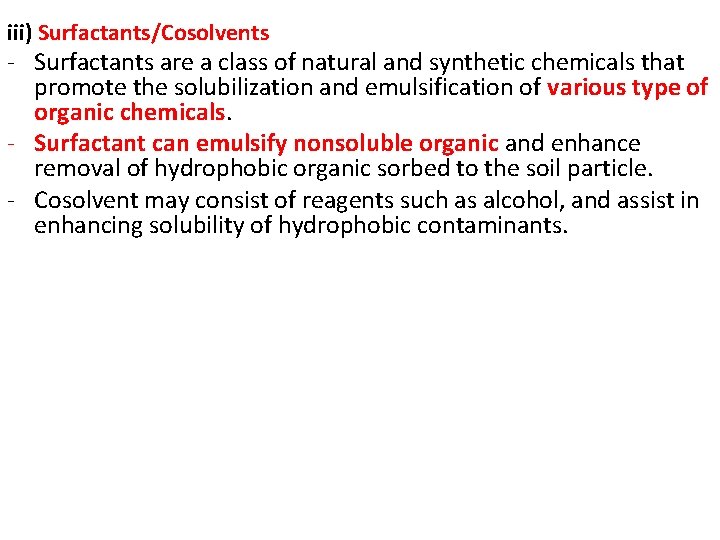
iii) Surfactants/Cosolvents - Surfactants are a class of natural and synthetic chemicals that promote the solubilization and emulsification of various type of organic chemicals. - Surfactant can emulsify nonsoluble organic and enhance removal of hydrophobic organic sorbed to the soil particle. - Cosolvent may consist of reagents such as alcohol, and assist in enhancing solubility of hydrophobic contaminants.

iv) Reductants/Oxidants - Reductants and oxidants can convert heavy metals to more soluble compounds.


• In the chemical oxidation, contaminated soil is mixed with an oxidation agent such as O 3, H 2 O 2, etc. • Redox reaction chemically convert hazardous waste contaminants into nonhazardous or less toxic compounds. • This method can be applied for contaminants such as inorganics, nonhaloginated VOC and SVOC, fuel hydrocarbons and pesticides. • Care should be taken to ensure that oxidation reactions do not form intermediate contaminants which may be more toxic than the parent compounds.

Types of flushing agent Flushing agent Contaminants Targeted Water High solubility organics; soluble inorganic salts Low solubility, hydrophobic organics; petroleum products Surfactants/ cosolvents Water/surfactant Medium solubility organic Acids Bases Reductants/ Oxidants Basic organic contaminants; metal Phenolics; metals Metals; organic contaminants

Limitation - Soil flushing is not feasible when complex wastes containing a range of contaminants with different solubility characteristic. Oil is poorly extracted by water. - The soil system after treatment is altered from it original state. Its physical, chemical and biological properties may be altered adversely; the p. H may be lowered by the used of acidic solvent; soil may become compacted. - The soil properties may have to be restored to ensure that other treatment process can occur such as bioremediation.

• Large volume of contaminated elutriate that require treatment. The recovered contaminants solution can be very dilute in a large volume and, hence, difficult to treat and disposal economically. • The solution used for flushing may themselves be potential pollutant. It need to ensure complete to recover of elutriate. • Soil flushing is less effective with contaminant that are relatively insoluble or tightly bound to soil. High clay content can cause adsorption of a surfactant. • Potential for spreading contaminants beyond the captured zone, vertically or horizontally, if the extraction system is not properly designed or constructed.

Advantages - Soil flushing is an in situ technology, the need to excavate, handle, and transport large quantity of the original contaminant is eliminated. - Potentially applicable to a wide range of contaminants in both vadose and saturated zones. - May be used in conjunction with other technologies.

Soil Washing • Soil washing is an ex situ process in which the contaminated soil is excavated and washed with water and water soluble cleansing agents. • The wastewater and rinsewater which now contain the contaminants are then treated. The clean soil then may be used as backfilled at the excavated location or landfill at another location. The soil is physically separated into various fraction and each fraction then cleansed to different degree based on reuse function or ultimate disposal requirement. • In some application, not all the separated size fractions are washed. If a separated soil fraction represents a small percentage of the total soil volume and most of the contaminants are sorbed in this fraction, then direct disposal of this fraction may be more economical.

• The concept of reducing soil contamination through the use of particle size separation is based on the finding that most organic and inorganic contaminants tend to bind, either chemically or physically, to clay, silt, and organic soil particles. • The silt and clay, in turn, are attached to sand gravel particles by physical processes, primarily compaction and adhesion. • Washing processes that separate the fine (small) clay and silt particles from the coarser sand gravel soil particles effectively separate and concentrate the contaminants into a smaller volume of soil that can be further treated or disposed of. • Soil washing is performed in containment vessel, which allow for process control and performance evaluation.


Soil Washing Process - Following excavation, the soil is screened to separate coarse debris (larger than about 2 in. ) such as rocks and roots. - The remaining soil may be fluidized, or made pumpable, with the addition of water. - In the scrubbing unit, a water-based washing solution is used to separate soluble contaminants and find particles from coarse soil materials. - Surficial contaminants is removed from the coarse fractions by solution and by the aggressive scouring action maintain in the unit - Dissolution of contaminants can be enhanced with chemical additives; for example, acidic wash solutions may be used to solubilize lead or other metal. Surfactants may also be added.

• Following washing, the soil slurry undergoes a separation step in which water, cleaned coarse materials, and contaminated fines materials are segregated. • Suspended fines may be flocculated and separated by gravity means or may be removed in a vacuum filter press. The clean soil in then returned to the excavation. • Typically, scrubbing solutions are at least partially recycled. Non-recycled solutions are treated using conventional wastewater treatment technologies. • The residuals from wastewater treatment (e. g spent exchange resins, spent carbon, or biological treatment sludge) may then be combined with the contaminated fines soils and sent for treatment or disposal.

• Treated soils are sampled and tested for their contaminants levels to verify that the remedial standards have been met. • Volatile compounds can also be collected from the soil handling and washing units and treated. • Air emissions can be controlled throughout the process. Typical air treatment process include activated carbon adsorption or catalytic or thermal oxidation.

Soil Feeding Clean Sand Product for on-site Backfill Soil Separation and washing

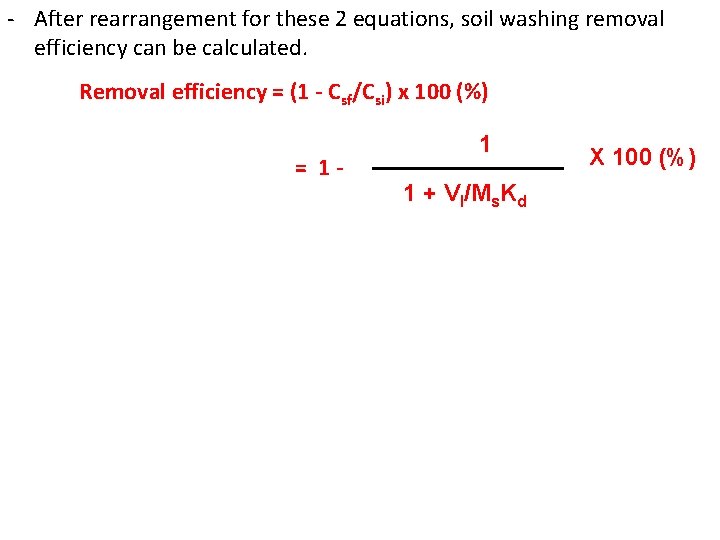
- At the end of soil washing, the majority of contaminants is transferred into washing solution; however some of the contaminants may remain in soil. Csi. Ms = Csf. Ms + Vl. Cl Csi = initial contaminant con. in soil (mg/kg) Ms = total dry mass of the soil (kg) Csf = final contaminants con. in soil (mg/kg) Vl = total volume of washing solution (L) Cl = contaminants con. in the washing solution (mg/L) Equilibrium condition will reached if the washing is continued for adequate time. At equilibrium, the contaminants con in washing solution (Cl) and in the soil (Csf) can be related by the distribution coefficient (Kd) of the contaminant between soil and washing solution. Csf = Kd. Cl

- After rearrangement for these 2 equations, soil washing removal efficiency can be calculated. Removal efficiency = (1 - Csf/Csi) x 100 (%) = 1 - 1 1 + Vl/Ms. Kd X 100 (%)

Applicability of Soil Washing - Soil washing can be effective for treating soil contaminated with a variety of organic and inorganic contaminants. - The target contaminant groups for soil washing are SVOCs, fuels, PAH, PCB, cyanides and heavy metals. The technology offers the ability for recovery of metals and can clean a wide range of organic and inorganic contaminants from coarsegrained soils. - Soil washing is less likely to be effective with silt or clay soil. The soil types for which this can be applied effectively are those with relatively high hydraulic conductivities. - If the contaminants adsorb to the soil strongly, soil washing may not be affective.

Merits of Soil washing Method - This process significantly reduces the volume of contaminated soil in that the contaminants are concentrated in a relatively small mass of materials. The soil washing would lead to a volume reduction of about 90% which means that only 10% of the original volume would require further treatment. - Closed system unaffected by external conditions. This system permits control of the conditions (such as p. H level and temperature) under which the soil particles are treated. - Allows the contaminated soil to be excavated and treated on site. Clean soils can be backfilled at the site. - Potential to remove both organic and inorganic contaminants.

Limitation of Soil Washing - Ineffective for soils containing 30 to 50% of silt, clay or organic matter. Contaminants tend to bind readily, chemically or physically, to silt, clay and organic materials. It may be difficult to remove organics adsorbed onto claysize particles. - Relatively expensive due to the additional costs associated with treating wastewater and air emissions. - Complex waste mixtures (e. g. , metals with organics) make formulating washing fluid difficult. - Small volumes of contaminated sludge and wash water remain at the end of the treatment, which require further treatment and disposal. - Exposure of the contaminants to the public is possible as a result of soil excavation and handling. - A large space is required based on the design of the soil washing system.

Soil vacuum extraction/soil vapor extraction (SVE) • It is a relatively simple process that physically separates contaminants from soil. SVE is applicable where soils are relatively homogeneous and highly permeable. • SVE extracts contaminants from the soil in vapor form. Therefore, SVE systems are designed to remove contaminants that have a tendency to volatilize or evaporate easily. SVE removes volatile organic compounds (VOCs) and some semi-volatile organic compounds (SVOCs) from soil below the ground surface in the unsaturated zone (vadose zone). • Vacuum is applied through extraction wells to create a pressure/concentration gradient that induces gas-phase volatiles to be removed from soil through extraction wells. • Often, in addition to vacuum extraction wells, air injection wells are installed to increase the air flow and improve the removal rate of the contaminant. An added benefit of introducing air into the soil is that it can stimulate bioremediation of some contaminants.


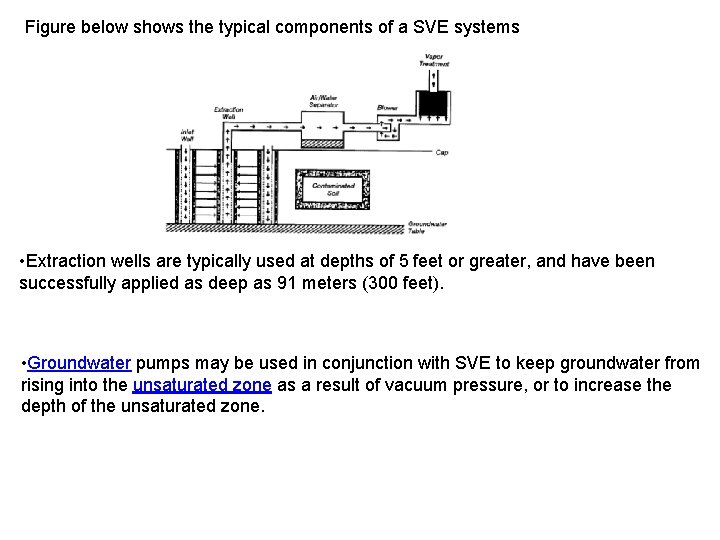
Figure below shows the typical components of a SVE systems • Extraction wells are typically used at depths of 5 feet or greater, and have been successfully applied as deep as 91 meters (300 feet). • Groundwater pumps may be used in conjunction with SVE to keep groundwater from rising into the unsaturated zone as a result of vacuum pressure, or to increase the depth of the unsaturated zone.

How does an SVE system work? • The first step to constructing an SVE system is to install vapor extraction wells and injection Wells (or air vents) in the contaminated area. Air injection wells use air compressors to force air into the ground. Air vents serve the same function as air injection wells, but are passive-instead of pumping air they just provide a passage for air to be drawn into the ground. When incoming air passes through the soil on its way to the extraction wells, contaminants evaporate out of the spaces between the soil particles and are pulled by the air to the wells and removed. • Vapor extraction wells can be placed either vertically or horizontally. Typically, they are placed vertically and are designed to penetrate the lower portion of the unsaturated zone. • Vapors extracted by the SVE process are typically treated using carbon adsorption, incineration, catalytic oxidation etc. Other methods, such as biological treatment and ultraviolet oxidation, also have been used with SVE systems. The type of treatment chosen depends on which contaminants are present and their concentrations. • Carbon adsorption is the most commonly used treatment for contaminated vapors and is adaptable to a wide range of volatile organic compounds. When properly designed and operated, SVE is a safe, low maintenance process.

Volatile Organic Compounds (VOCs) • One of a group of carbon-containing compounds that evaporate readily at room temperature. Examples of VOCs include trichloroethane, trichloroethylene, benzene, toluene, ethylbenzene, and xylene (BTEX). Semi-Volatile Organic Compounds (SVOCs) • Substances composed primarily of carbon and hydrogen atoms that have boiling points greater than 2000 C. Common SVOCs include PCBs and PAHs.


Initial Screening Of SVE Effectiveness • The key parameters that should be used to decide whether SVE will be a viable remedy for a particular site are: • Permeability of the petroleum-contaminated soils. Permeability of the soil determines the rate at which soil vapors can be extracted. • Volatility of the petroleum constituents. Volatility determines the rate (and degree) at which petroleum constituents will vaporize from the soil-adsorbed state to the soil vapor state



Evaluation of SVE Effectiveness Evaluation is based on the permeability of the soil and the volatility of the constituents. Parameters for soil permeability: Intrinsic permeability Soil structure and stratification Depth to ground water Moisture content Parameters for constituent volatility: Vapor pressure Product composition and boiling points Henry’s law constant

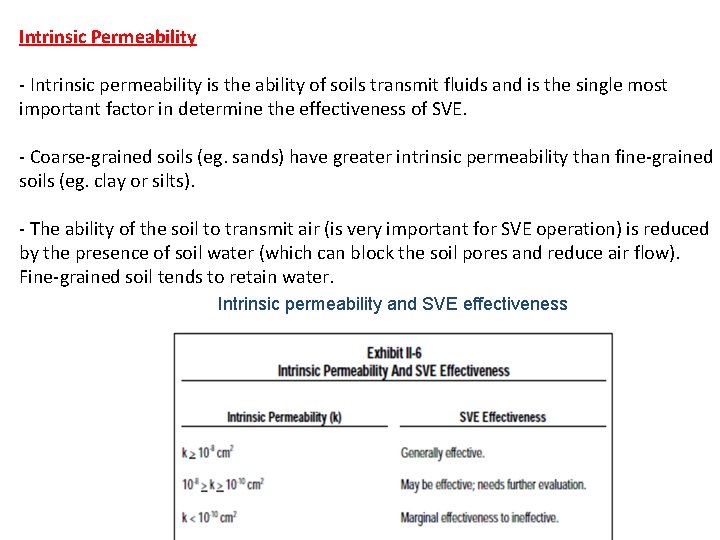
Intrinsic Permeability - Intrinsic permeability is the ability of soils transmit fluids and is the single most important factor in determine the effectiveness of SVE. - Coarse-grained soils (eg. sands) have greater intrinsic permeability than fine-grained soils (eg. clay or silts). - The ability of the soil to transmit air (is very important for SVE operation) is reduced by the presence of soil water (which can block the soil pores and reduce air flow). Fine-grained soil tends to retain water. Intrinsic permeability and SVE effectiveness

Soil structure and stratification - It can affect how and where soil vapors will flow within the soil matrix under extraction condition. - Structural characteristic such as microfracturing can result in higher permeabilities than expected for certain soil components (eg. Clay). However, the increased flow ability will be confined within fractures but not in the unfractured media. This flow behavior leads to ineffective extraction and extended remedial times. - Stratification of soils with different permeabilities can increase the lateral flow of soil vapors in the more permeable stratum while reducing in the less permeable stratum.

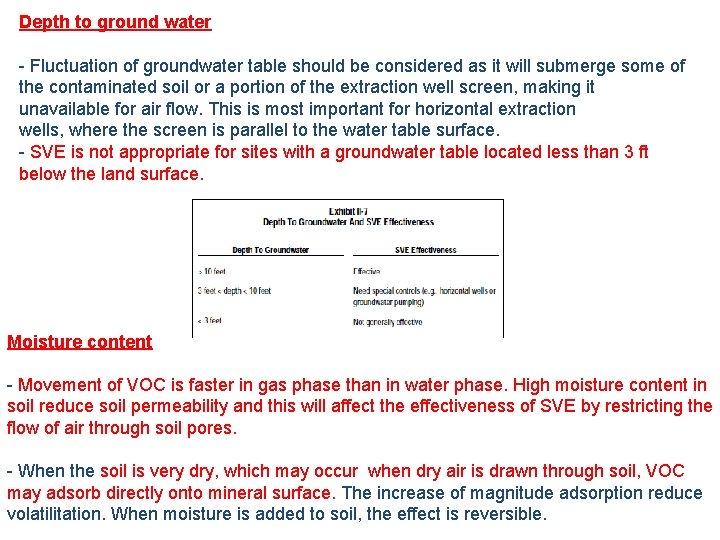
Depth to ground water - Fluctuation of groundwater table should be considered as it will submerge some of the contaminated soil or a portion of the extraction well screen, making it unavailable for air flow. This is most important for horizontal extraction wells, where the screen is parallel to the water table surface. - SVE is not appropriate for sites with a groundwater table located less than 3 ft below the land surface. Moisture content - Movement of VOC is faster in gas phase than in water phase. High moisture content in soil reduce soil permeability and this will affect the effectiveness of SVE by restricting the flow of air through soil pores. - When the soil is very dry, which may occur when dry air is drawn through soil, VOC may adsorb directly onto mineral surface. The increase of magnitude adsorption reduce volatilitation. When moisture is added to soil, the effect is reversible.

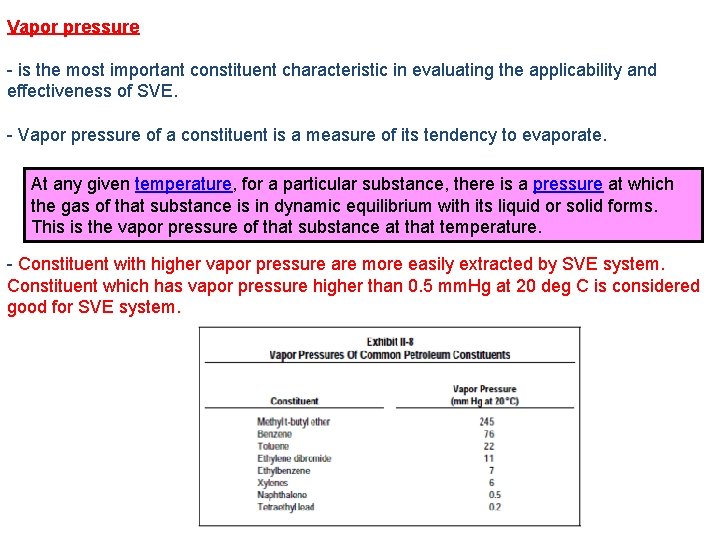
Vapor pressure - is the most important constituent characteristic in evaluating the applicability and effectiveness of SVE. - Vapor pressure of a constituent is a measure of its tendency to evaporate. At any given temperature, for a particular substance, there is a pressure at which the gas of that substance is in dynamic equilibrium with its liquid or solid forms. This is the vapor pressure of that substance at that temperature. - Constituent with higher vapor pressure are more easily extracted by SVE system. Constituent which has vapor pressure higher than 0. 5 mm. Hg at 20 deg C is considered good for SVE system.

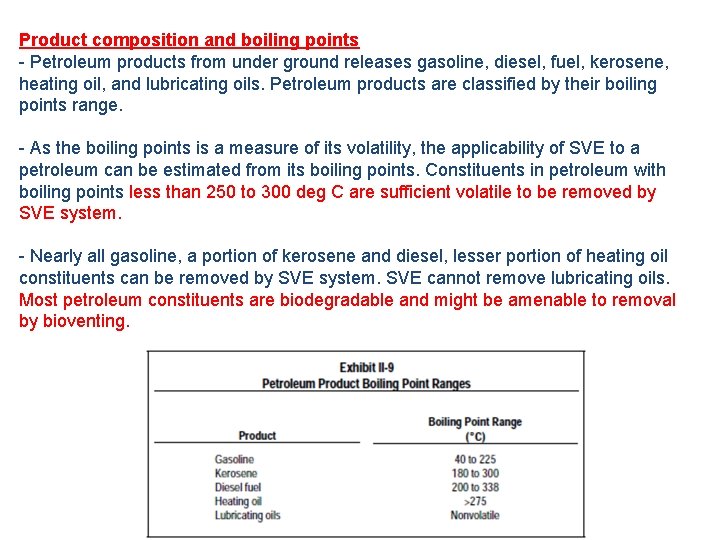
Product composition and boiling points - Petroleum products from under ground releases gasoline, diesel, fuel, kerosene, heating oil, and lubricating oils. Petroleum products are classified by their boiling points range. - As the boiling points is a measure of its volatility, the applicability of SVE to a petroleum can be estimated from its boiling points. Constituents in petroleum with boiling points less than 250 to 300 deg C are sufficient volatile to be removed by SVE system. - Nearly all gasoline, a portion of kerosene and diesel, lesser portion of heating oil constituents can be removed by SVE system. SVE cannot remove lubricating oils. Most petroleum constituents are biodegradable and might be amenable to removal by bioventing.

Henry’s law constant - Is a indicator of the volatility of a constituents. - Henry’s law constant is the partitioning coefficient that relates the concentration of a constituent dissolved in water to its partial pressure in the vapor phase under equilibrium conditions. KH = Pgas/ Cequil = conc of a gas dissolved in liquid at equilibrium Pgas = partial pressure of gas above liquid KH = Henry’s Law constant for a gas at a given temperature - It describes the relative tendency for a dissolved constituent to partition between the vapor phase and the dissolved phase. Thus, the Henry’s law constant is a measure of the degree for the constituents that are dissolved in soil moisture (or ground water) will volatile for removal by SVE system.

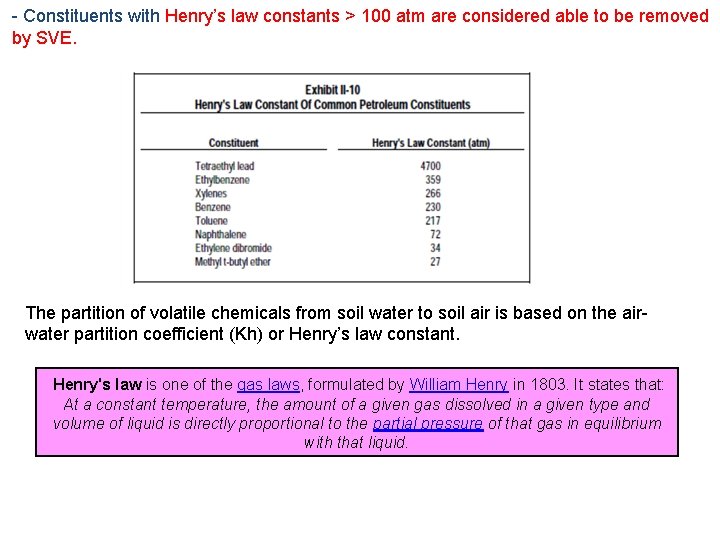
- Constituents with Henry’s law constants > 100 atm are considered able to be removed by SVE. The partition of volatile chemicals from soil water to soil air is based on the airwater partition coefficient (Kh) or Henry’s law constant. Henry's law is one of the gas laws, formulated by William Henry in 1803. It states that: At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.

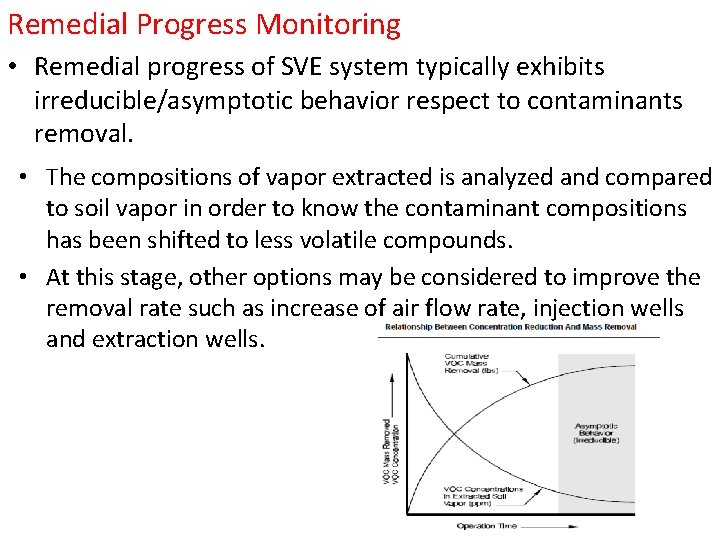
Remedial Progress Monitoring • Remedial progress of SVE system typically exhibits irreducible/asymptotic behavior respect to contaminants removal. • The compositions of vapor extracted is analyzed and compared to soil vapor in order to know the contaminant compositions has been shifted to less volatile compounds. • At this stage, other options may be considered to improve the removal rate such as increase of air flow rate, injection wells and extraction wells.