Soft Materials n n Toothpaste Detergent By Jennifer

Soft Materials n n Toothpaste Detergent By: Jennifer, Jackie, Nicole, Dustin and Steve

• Cells are held intact by the 'cytoskeleton', a network of protein fibres inside the cell. Ben Fabry, from the Harvard School of Public Health in Boston, and colleagues measured how this scaffolding deforms under a range of forces by attaching tiny magnetic beads to cytoskeletons, and applying an oscillating magnetic field 1. • Just as toothpaste stays solid in its tube, until given enough of a squeeze that it flows obligingly onto your toothbrush, so the cytoskeleton holds together like a solid most of the time. But under the right conditions, it transforms easily into something more like a fluid, accommodating expansion, contraction or division. • So toothpaste would be considered a “SGM” (soft glassy material)


P&G's impact on dental health has been immense. The introductions of Crest and later Crest Tartar Control have helped reduce the average of missing teeth or cavities in the United States from 15 to three, with more than 50 percent of children today having no tooth decay.
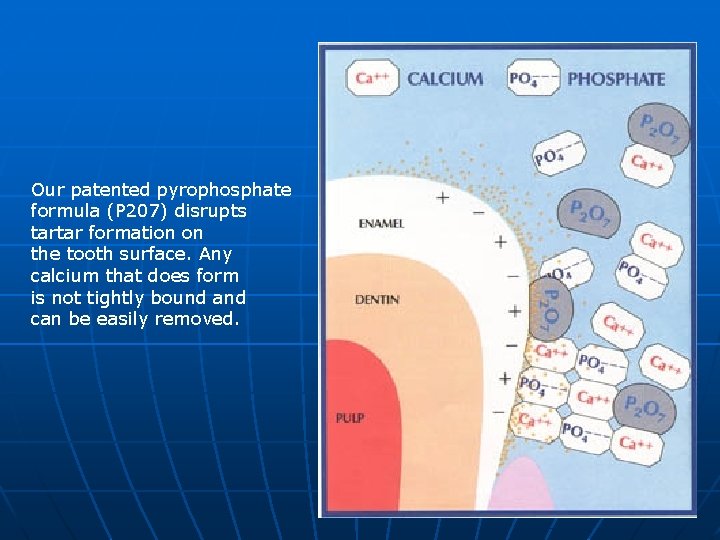
Our patented pyrophosphate formula (P 207) disrupts tartar formation on the tooth surface. Any calcium that does form is not tightly bound and can be easily removed.
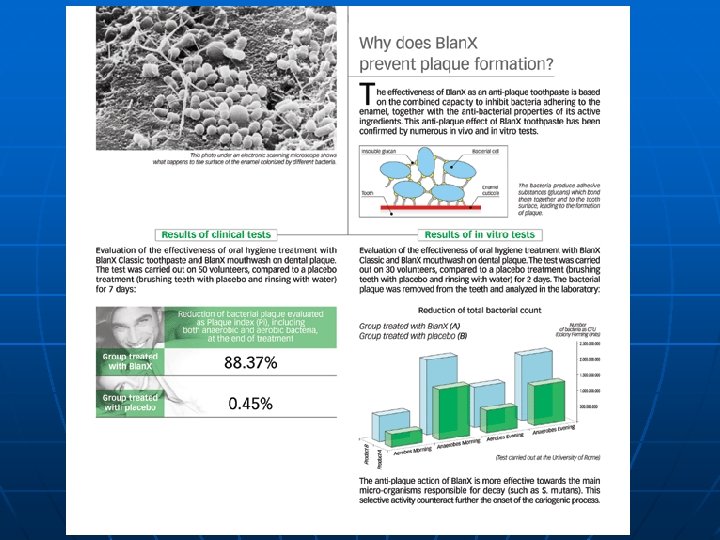

Detergent n n A detergent is a compound, or a mixture of compounds, intended to assist cleaning. They lower the surface tension of a liquid allowing easier spreading and the interfacial tension between two liquids.

How Does It Work? n Soaps and detergents are made from long molecules that contain a head and tail. These molecules are called surfactants; the diagram below represents a surfactant molecule. n n n The head of the molecule is attracted to water (hydrophilic) and the tail is attracted to grease and dirt (hydrophobic). When the detergent molecules meet grease on clothes, the tails are drawn into the grease but the heads still sit in the water. The attractive forces between the head groups and the water are so strong that the grease is lifted away from the surface. The blob of grease is now completely surrounded by detergent molecules and is broken into smaller pieces which are washed away by the water
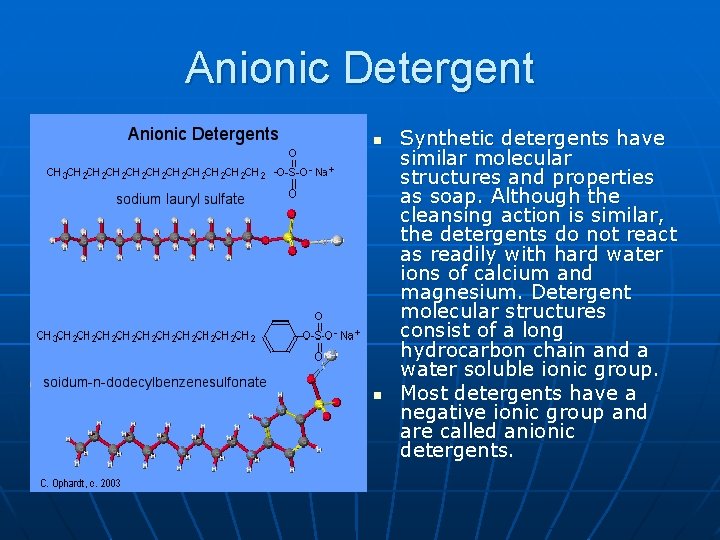
Anionic Detergent n n Synthetic detergents have similar molecular structures and properties as soap. Although the cleansing action is similar, the detergents do not react as readily with hard water ions of calcium and magnesium. Detergent molecular structures consist of a long hydrocarbon chain and a water soluble ionic group. Most detergents have a negative ionic group and are called anionic detergents.
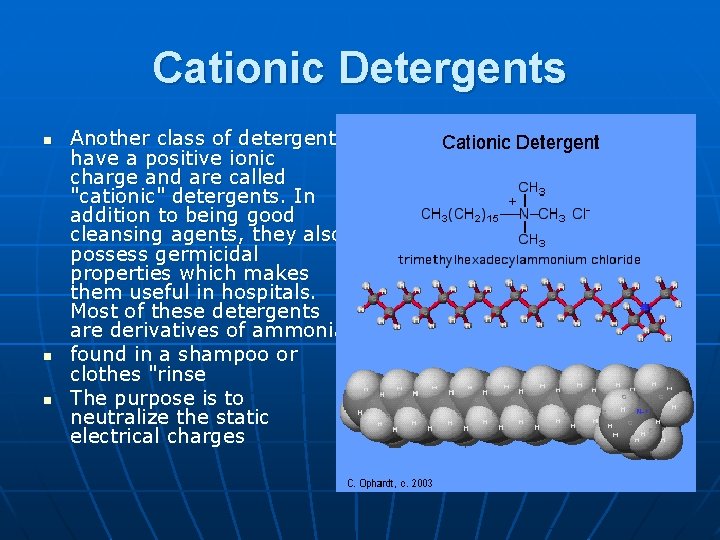
Cationic Detergents n n n Another class of detergents have a positive ionic charge and are called "cationic" detergents. In addition to being good cleansing agents, they also possess germicidal properties which makes them useful in hospitals. Most of these detergents are derivatives of ammonia. found in a shampoo or clothes "rinse The purpose is to neutralize the static electrical charges

Neutral or non-ionic detergents Nonionic detergents are used in dish washing liquids. Since the detergent does not have any ionic groups, it does not react with hard water ions. In addition, nonionic detergents foam less than ionic detergents. The detergent molecules must have some polar parts to provide the necessary water solubility.

- Slides: 13