Soft Capsules Soft gelatin capsules are made of










- Slides: 18

Soft Capsules

Soft gelatin capsules are made of gelatin to which glycerin or a polyhydric alcohol such as sorbitol has been added. Properties of Soft Gelatin Shells Soft gelatin capsules, which contain more moisture than hard capsules, may have a preservative, such as methylparaben and/or propylparaben, to retard microbial growth

Methods of Manufacturing • Soft gelatin capsules are manufactured by four methods: 1) Plate press method 2) Rotary die process 3) Accogel process 4) Bubble method

1) Plate Press Method • Semi-automatic batch process. PROCEDURE : • Place the upper half of a plasticized gelatin sheet over a die plate containing numerous die pockets. • Application of vacuum to draw the sheet into die pockets. • Filling the pockets with liquid or paste. • Folding the lower half of the gelatin sheet back over the filled pockets. • Inserting the sandwich under a die press where the capsules are formed and cut out.

• The material to be encapsulated flows by gravity. • The gelatin sheets are feed on rolls contain small orifice lined up with the die pocket of the die roll. • Two plasticized gelatin ribbons are continuously and simultaneously fed with the liquid or paste fill between the rollers of the rotary die mechanism where the capsule are simultaneously filled, shaped, hermetically sealed and cut from the gelatin ribbon. 2) Rotary die process • The sealing of the capsule is achieved by mechanical pressure on the die rolls and the heating(37 -40°C) of the ribbons by the wedge.


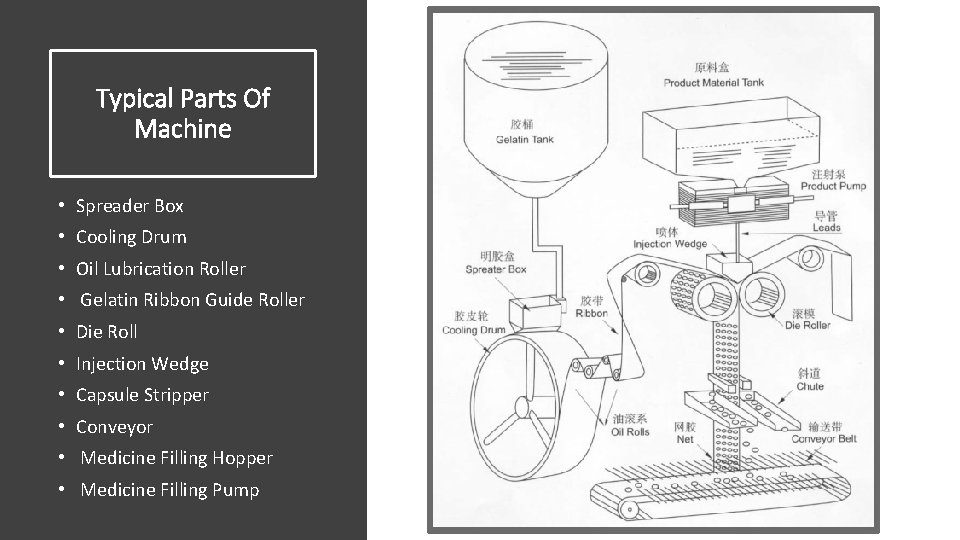
Typical Parts Of Machine • Spreader Box • Cooling Drum • Oil Lubrication Roller • Gelatin Ribbon Guide Roller • Die Roll • Injection Wedge • Capsule Stripper • Conveyor • Medicine Filling Hopper • Medicine Filling Pump

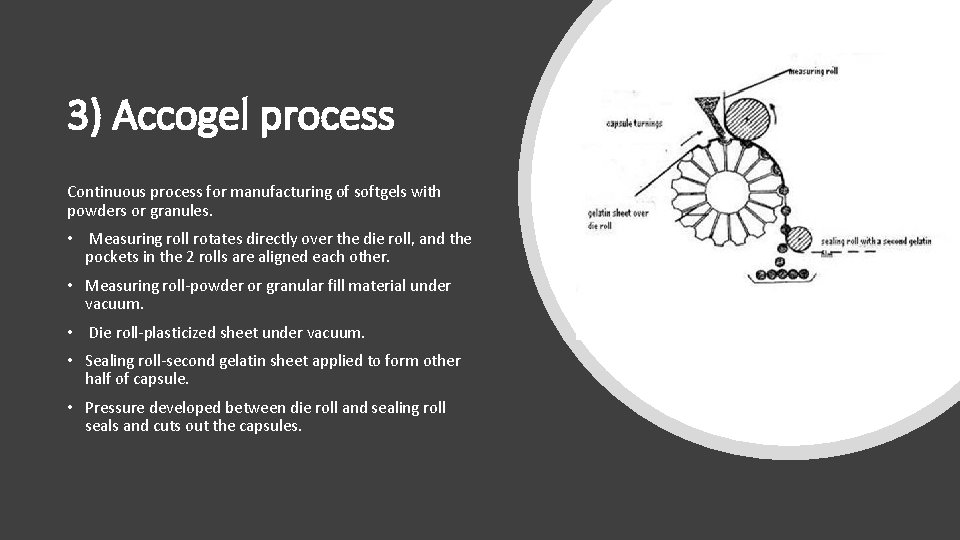
3) Accogel process Continuous process for manufacturing of softgels with powders or granules. • Measuring roll rotates directly over the die roll, and the pockets in the 2 rolls are aligned each other. • Measuring roll-powder or granular fill material under vacuum. • Die roll-plasticized sheet under vacuum. • Sealing roll-second gelatin sheet applied to form other half of capsule. • Pressure developed between die roll and sealing roll seals and cuts out the capsules.

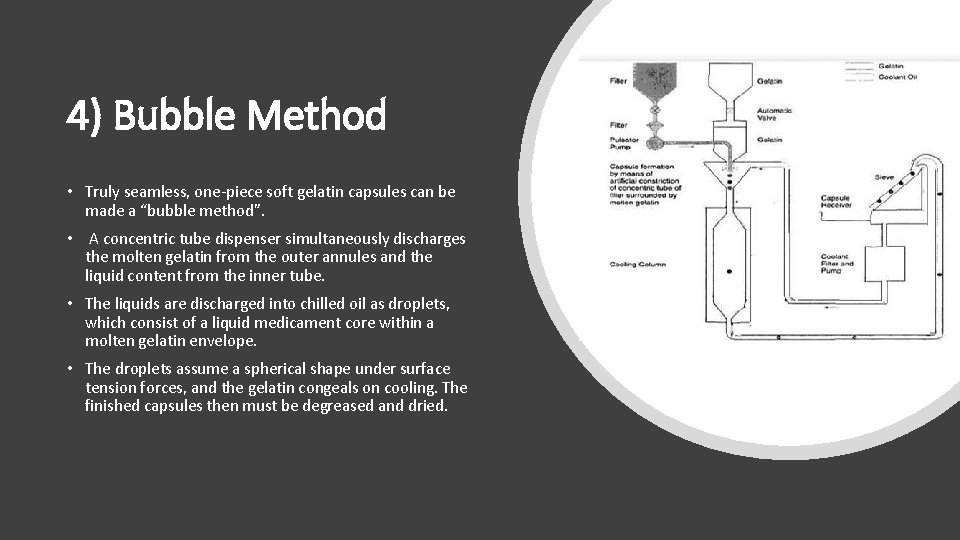
4) Bubble Method • Truly seamless, one-piece soft gelatin capsules can be made a “bubble method”. • A concentric tube dispenser simultaneously discharges the molten gelatin from the outer annules and the liquid content from the inner tube. • The liquids are discharged into chilled oil as droplets, which consist of a liquid medicament core within a molten gelatin envelope. • The droplets assume a spherical shape under surface tension forces, and the gelatin congeals on cooling. The finished capsules then must be degreased and dried.

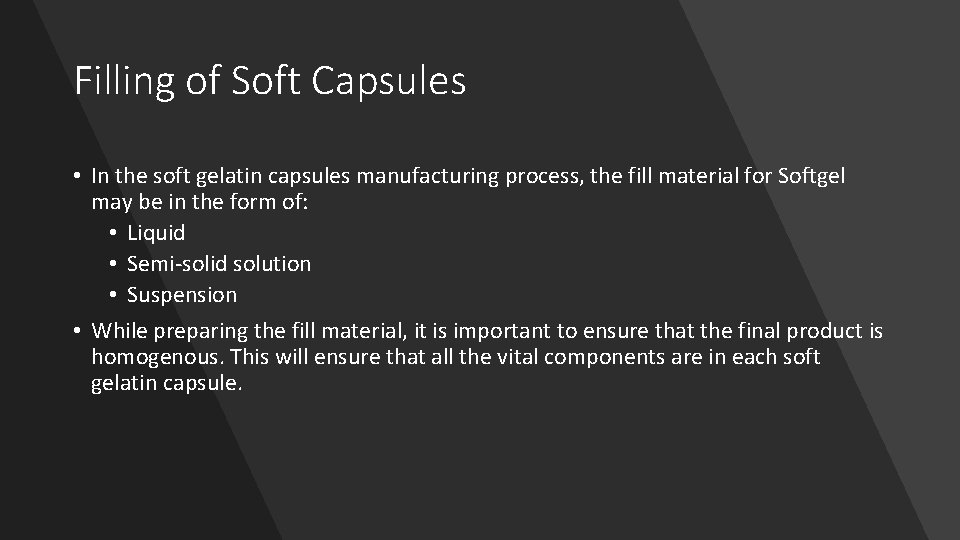
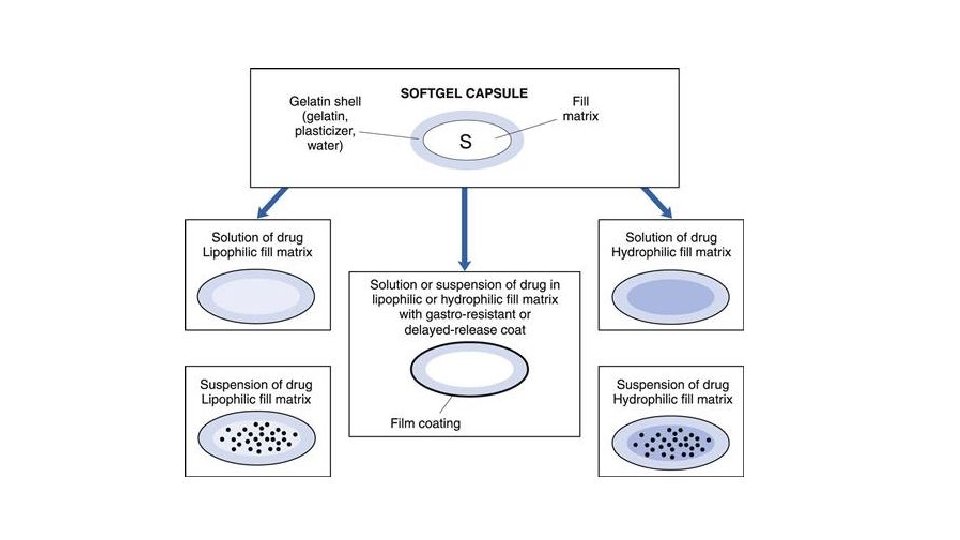
Filling of Soft Capsules • In the soft gelatin capsules manufacturing process, the fill material for Softgel may be in the form of: • Liquid • Semi-solid solution • Suspension • While preparing the fill material, it is important to ensure that the final product is homogenous. This will ensure that all the vital components are in each soft gelatin capsule.


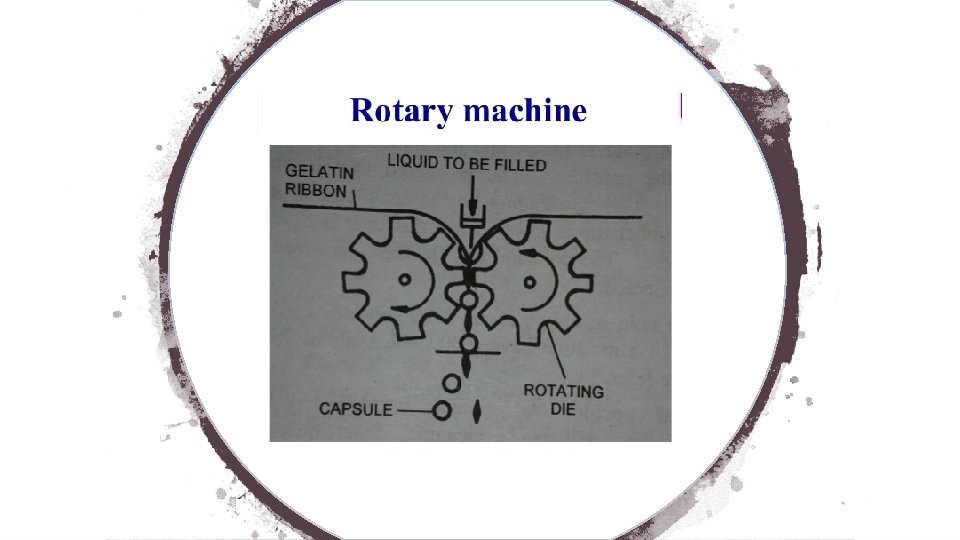
• Soft gelatin capsules are generally filled mechanically. • The manufacturing of the capsule shell and the filling of the medicament take place simultaneously. • Nowadays, a rotary machine is used for this purpose. • In a rotary die machine, the soft gelatin capsules are prepared and then filled immediately with the liquid medicaments. • The machine consists of two hoppers. • Liquid gelatin mixture is placed in one hopper and the liquid medicament in the other hopper. • There are two rotating dies which rotate in opposite directions.


Formulation Factors affecting drug availability in capsules 1) ADDED SUBSTANCES • Substances added to official preparations, including capsules, to enhance their stability, usefulness, or elegance or to facilitate their manufacture may be used only if they: • 1. Are harmless in the quantities used • 2. Do not exceed the minimum amounts required to provide their intended effect • 3. Do not impair the product’s bioavailability, therapeutic efficacy, or safety • 4. Do not interfere with requisite compendial assays and tests 2) CONTAINERS FOR DISPENSING CAPSULES • Depending on the item, the container may be required to be tight, well-closed, light resistant, and/or all of these.

3) DISINTEGRATION TEST FOR CAPSULES • The capsules are placed in the basket rack assembly, which is immersed 30 times per minute into a thermostatically controlled fluid at 37°C and observed. 4) Weight variation • The gross weight of 10 intact capsules is deter- mined individually. Then each capsule is cut open and the contents are removed by washing with a suitable solvent. The solvent is allowed to evaporate at room temperature over about 30 minutes, with precautions to avoid uptake or loss of moisture. The individual shells are weighed and the net contents calculated. From the results of the assay directed in the individual monograph, the content of the active ingredient in each of the capsules is determined. 5) Content uniformity 6) Dissolution test

Thank You !

Done by: • Rahaf Saeed Halawani (437000764) • Khadijah Ahmed Alzahrani (436002468) • Taif Saeed Alzahrani (437002160) • Hadeel Abdulhamid Alkediwi (436003784) • Raghad Hussien Foudah (437003972) • Moudhi Suliman Alkhalaf (437001186)

References Jones BE. Hard gelatin capsules and the pharmaceutical formulator. Pharm Technol 1985; 9: 106– 112. Digenis A, Gold TB, Shah VP. Crosslinking of gelatin capsules and its relevance to their in vitro/in vivo performance. Dissolut Tech 1995; 2: 1. Gardner D, Casper R, Leith F, et al. Noninvasive methodology for assessing regional drug absorption from the gastrointestinal tract. Pharm Technol 1997; 21: 82– 89. Wilding IR. Pharmacoscintigraphic evaluation of oral delivery systems, part I. Pharm Technol 1995; 19: 54– 60. Mojaverian P, Reynolds JC, Ouyang A, et al. Mechanism of gastric emptying of a nondisintegrating radiotelemetry capsule in man. Pharm Res 1991; 8: 97– 100. Nash RA. The “rule of sixes” for filling hard-shell gelatin capsules. Int Pharm Compound 1997; 1: 40 – 41. Yalkowsky SH, Bolton S. Particle size and content uniformity. Pharm Res 1990; 7: 962– 966. Swarbrick J. In vitro dissolution, drug bioavailability, and the spiral of science. Pharm Tech 1997; 21: 68– 72.