Soaps and Emulsions Overview In this section learn













- Slides: 24

Soaps and Emulsions Overview In this section, learn about the chemistry of soap-making, find about how soaps and detergents clean, and study the chemistry of emulsions and emulsifiers.

a) Making soap Learning intention Find out how soaps are formed by alkaline hydrolysis of fats and oils.

Soap formation This animation describes the formation of soap by the alkaline hydrolysis of fats / oils followed by neutralisation to form sodium salts of fatty acids.

Making Soaps

Thinking time • Draw the chemical structure of the chemicals that have reacted (general shape of oil will be enough). • Predict what you think the structure of soap might look like (baring in mind what we were doing with esters). • How does the soap clean dirt (particularly greasy things)

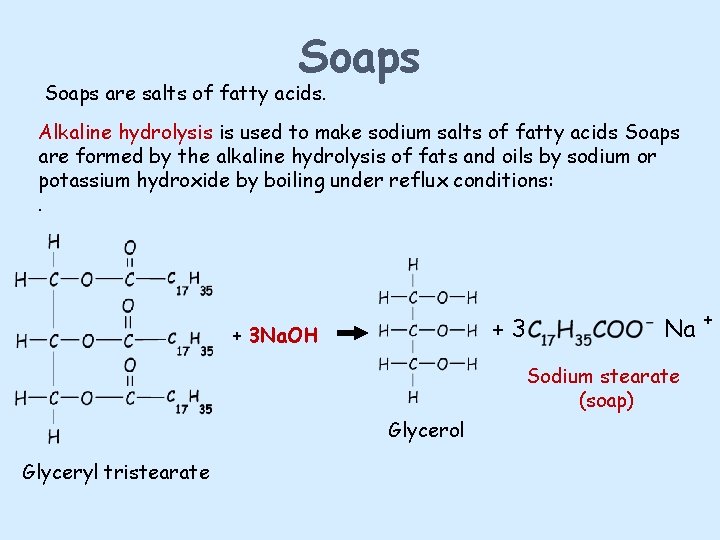
Soaps are salts of fatty acids. Alkaline hydrolysis is used to make sodium salts of fatty acids Soaps are formed by the alkaline hydrolysis of fats and oils by sodium or potassium hydroxide by boiling under reflux conditions: . +3 + 3 Na. OH Na + Sodium stearate (soap) Glycerol Glyceryl tristearate

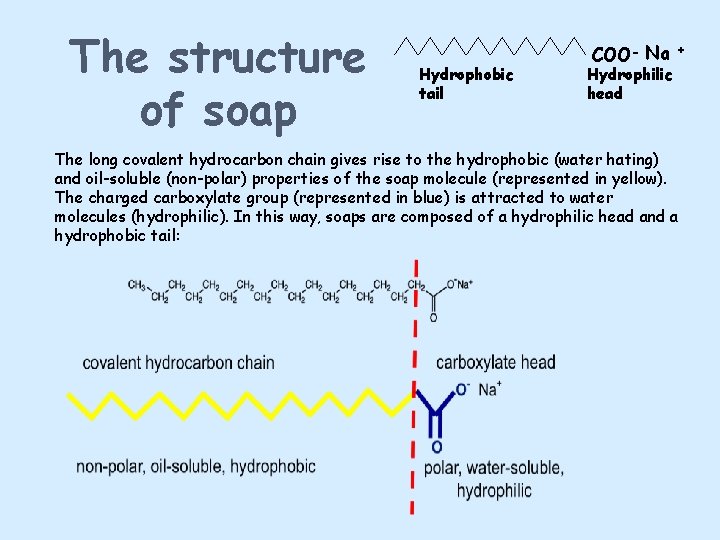
The structure of soap Hydrophobic tail COO- Na + Hydrophilic head The long covalent hydrocarbon chain gives rise to the hydrophobic (water hating) and oil-soluble (non-polar) properties of the soap molecule (represented in yellow). The charged carboxylate group (represented in blue) is attracted to water molecules (hydrophilic). In this way, soaps are composed of a hydrophilic head and a hydrophobic tail:

b) Cleansing action of soap Learning intention Learn how the characteristic structure of soap and detergent molecules allows effective cleaning of oily stains to take place.

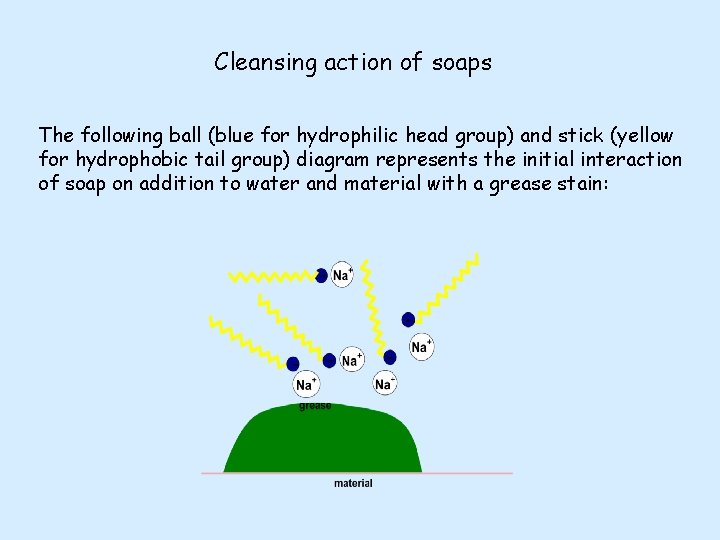
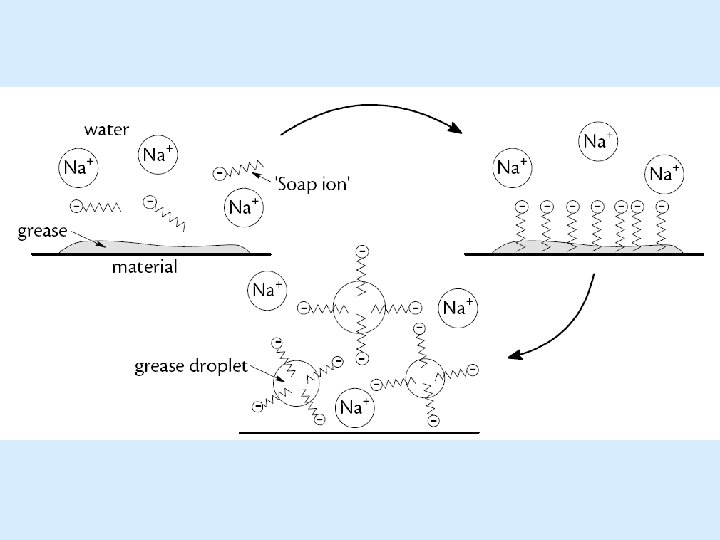
Cleansing action of soaps The following ball (blue for hydrophilic head group) and stick (yellow for hydrophobic tail group) diagram represents the initial interaction of soap on addition to water and material with a grease stain:

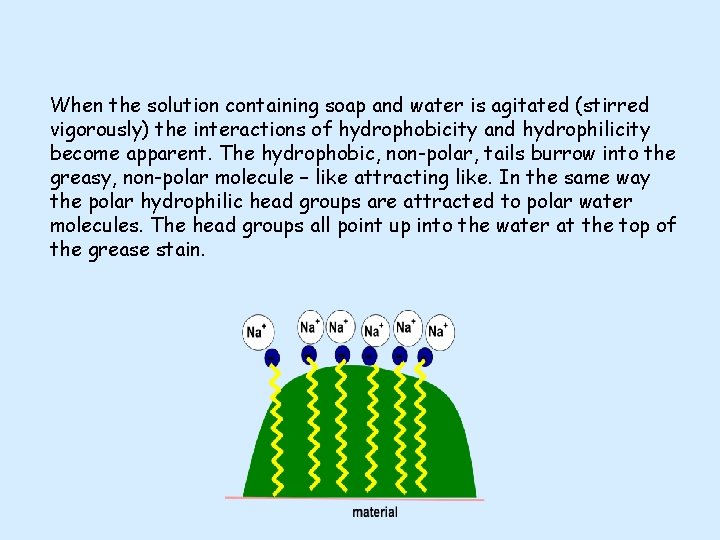
When the solution containing soap and water is agitated (stirred vigorously) the interactions of hydrophobicity and hydrophilicity become apparent. The hydrophobic, non-polar, tails burrow into the greasy, non-polar molecule – like attracting like. In the same way the polar hydrophilic head groups are attracted to polar water molecules. The head groups all point up into the water at the top of the grease stain.

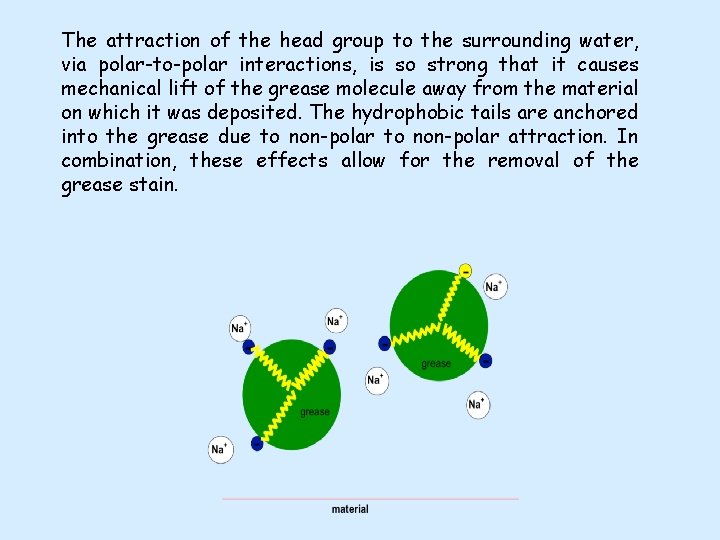
The attraction of the head group to the surrounding water, via polar-to-polar interactions, is so strong that it causes mechanical lift of the grease molecule away from the material on which it was deposited. The hydrophobic tails are anchored into the grease due to non-polar attraction. In combination, these effects allow for the removal of the grease stain.


• Soaps are produced by the alkaline hydrolysis of edible fats and edible oils. Hydrolysis produces three fatty acid molecules and one glycerol molecule. The fatty acid molecules are neutralised by the alkali, forming watersoluble, ionic salts called soaps. • Soaps can be used to remove non-polar substances such as oil and grease. Soap ions have long non-polar tails, readily soluble in non-polar compounds (hydrophobic), and ionic heads that are water-soluble (hydrophilic). The hydrophobic tails dissolve in the oil or grease. The negatively-charged hydrophilic heads remain in the surrounding water. • Agitation causes ball-like structures to form. The negativelycharged ball-like structures repel each other and the oil or grease is kept suspended in the water. • Hard water is a term used to describe water containing high levels of dissolved metal ions. • When soap is used in hard water, scum, an insoluble precipitate, is formed.

http: //www. educationscotland. gov. uk/highersciences/chemis try/animations/cleansingsoap. a sp

Starter


c) Emulsions in food Learning intention Learn about the characteristics of an emulsion, and study the chemistry of typical emulsifier molecules.

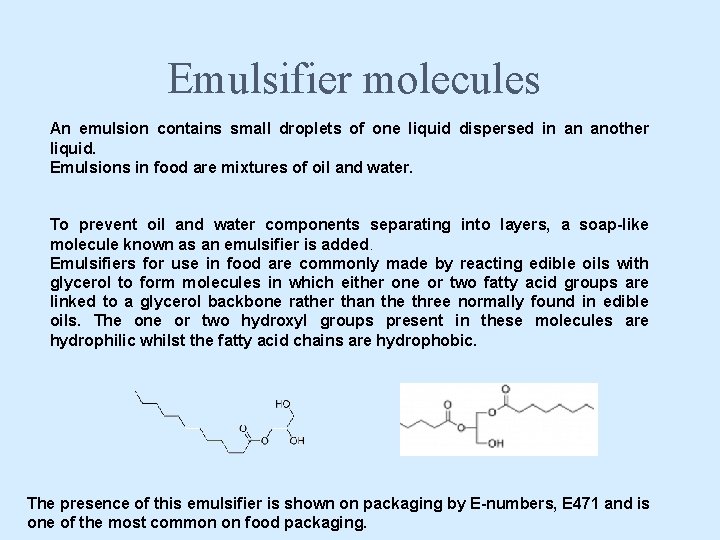
Emulsifier molecules An emulsion contains small droplets of one liquid dispersed in an another liquid. Emulsions in food are mixtures of oil and water. To prevent oil and water components separating into layers, a soap-like molecule known as an emulsifier is added. Emulsifiers for use in food are commonly made by reacting edible oils with glycerol to form molecules in which either one or two fatty acid groups are linked to a glycerol backbone rather than the three normally found in edible oils. The one or two hydroxyl groups present in these molecules are hydrophilic whilst the fatty acid chains are hydrophobic. The presence of this emulsifier is shown on packaging by E-numbers, E 471 and is one of the most common on food packaging.

Emulsifiers Mayonnaise contains oil and water. The emulsifier keeps these mixed and without it the oil and water separate.

Emulsifiers in food Emulsifiers are among the most frequently used types of food additives. They are used for many reasons. Emulsifiers can help to make a food appealing. They are used to aid in the processing of foods and also to help maintain quality and freshness. In low fat spreads, emulsifiers can help to prevent the growth of moulds which would happen if the oil and fat separated.

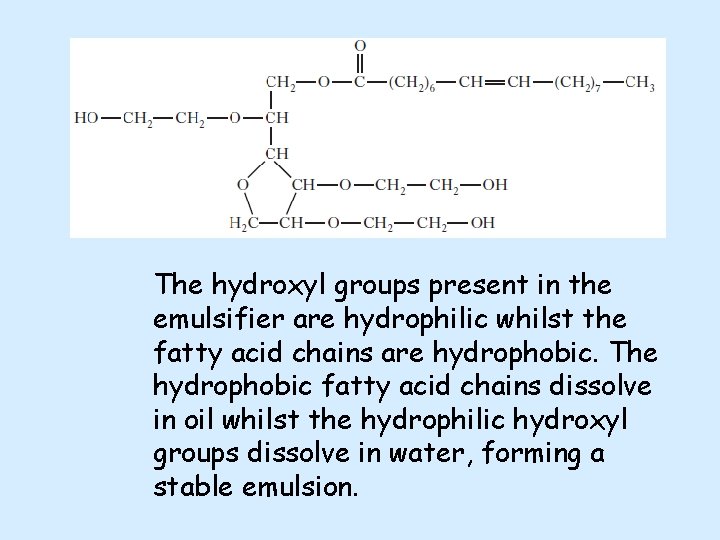
The hydroxyl groups present in the emulsifier are hydrophilic whilst the fatty acid chains are hydrophobic. The hydrophobic fatty acid chains dissolve in oil whilst the hydrophilic hydroxyl groups dissolve in water, forming a stable emulsion.

• An emulsifier can be used to prevent non-polar and polar liquids separating into layers. • An emulsion contains small droplets of one liquid dispersed in another liquid. • Emulsifiers for use in food can be made by reacting edible oils with glycerol. In the molecules formed, only one or two fatty acid groups are linked to each glycerol backbone.

Emulsifiers in food Foods that Commonly Contain Emulsifiers Biscuits Toffees Bread Extruded snacks Chewing gum Margarine / low fat spreads Breakfast cereals Frozen desserts Coffee whiteners Cakes Ice-cream Topping powders Desserts / mousses Dried potato Peanut butter Soft drinks Chocolate coatings Caramels

http: //www. educationscotland. gov. uk/higherscienc es/chemistry/animations/emulsions. asp This animation explains the difference between a stable and an unstable emulsion, and goes on to show addition of an emulsifier can stabilise an emulsion which is otherwise unstable. The chemical structure of a typical emulsifier is described and this is used to explain the favourable properties of emulsifiers