Soap and Detergents Fat or Oil Fatty acids








- Slides: 32

Soap and Detergents

Fat or Oil

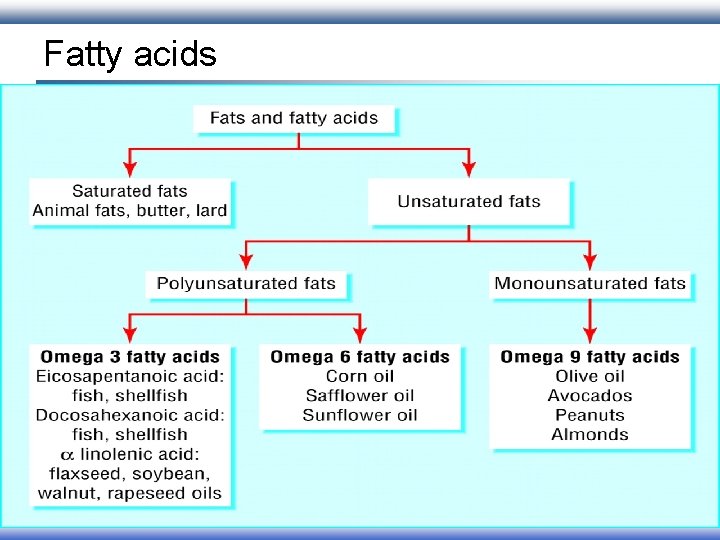
Fatty acids

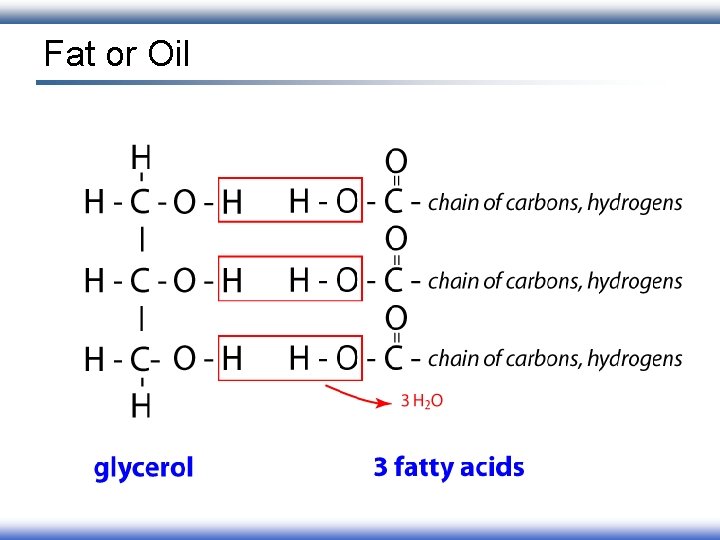
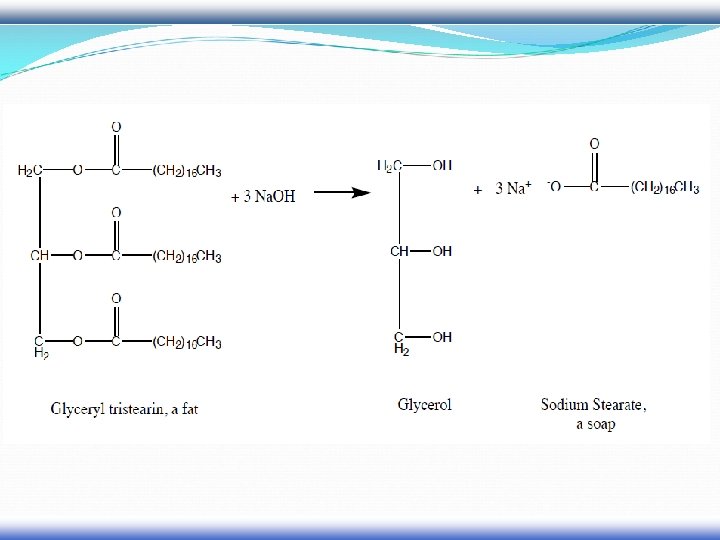
Fat or Oil

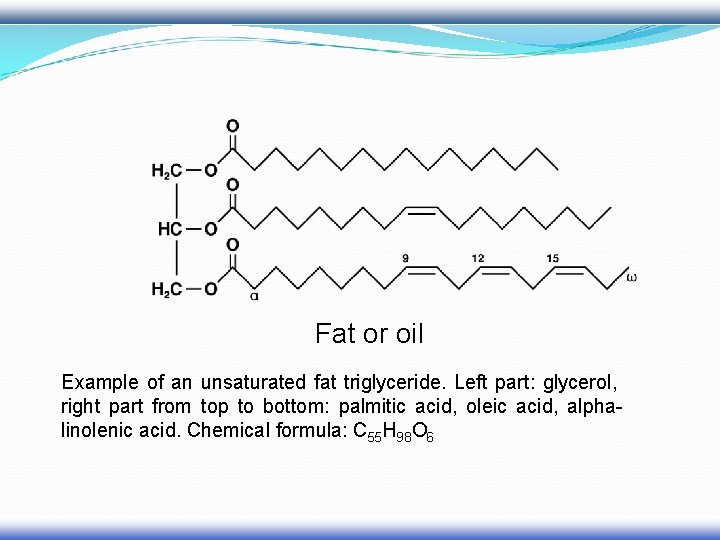
Fat or oil Example of an unsaturated fat triglyceride. Left part: glycerol, right part from top to bottom: palmitic acid, oleic acid, alphalinolenic acid. Chemical formula: C 55 H 98 O 6


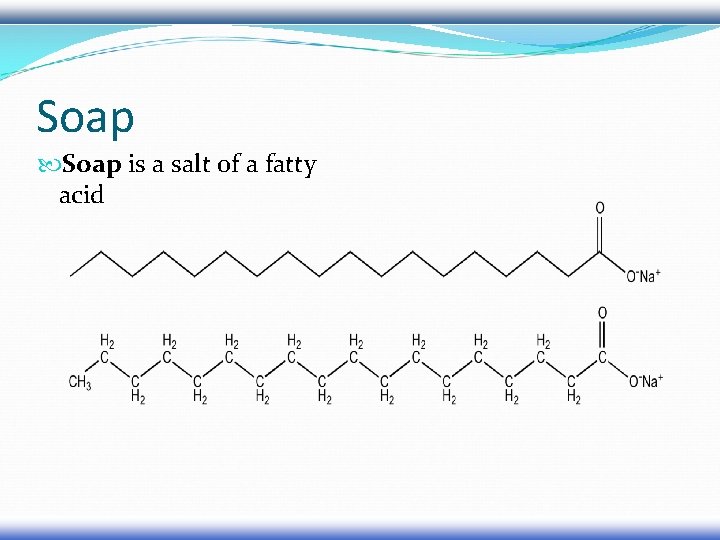
Soap is a salt of a fatty acid

Soaps are less effective in hard water, which is water that contains a significant concentration of Mg 2+ and Ca 2+ ions. These ions form precipitates with soap molecules, and this precipitate is often seen as a gray line on a bathtub or sink and is often called “soap scum”.

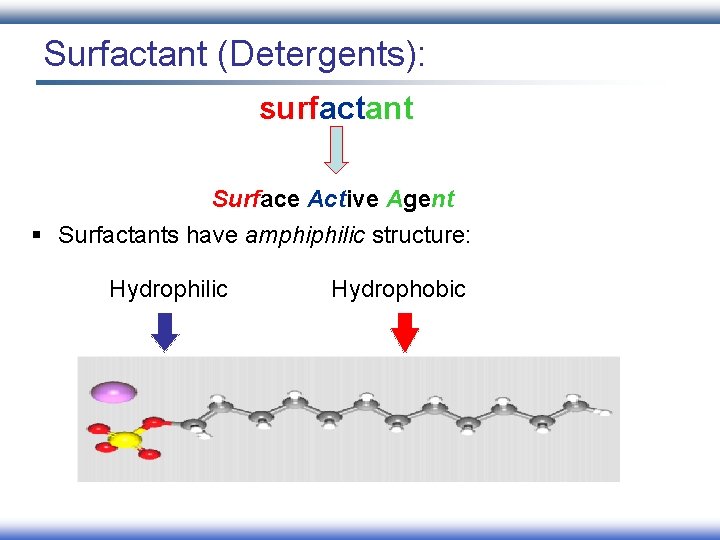
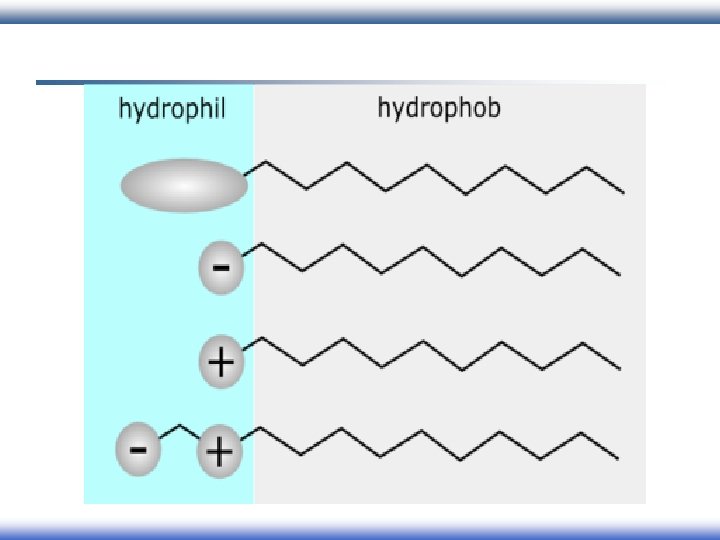
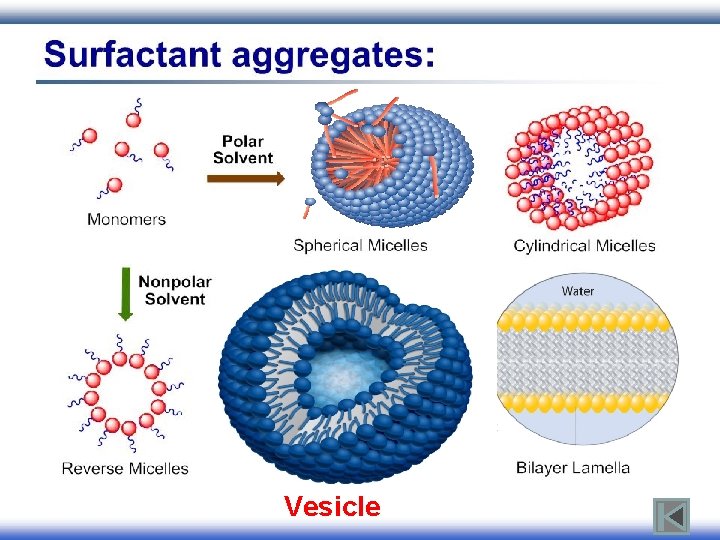
Surfactant (Detergents): surfactant Surface Active Agent § Surfactants have amphiphilic structure: Hydrophilic Hydrophobic

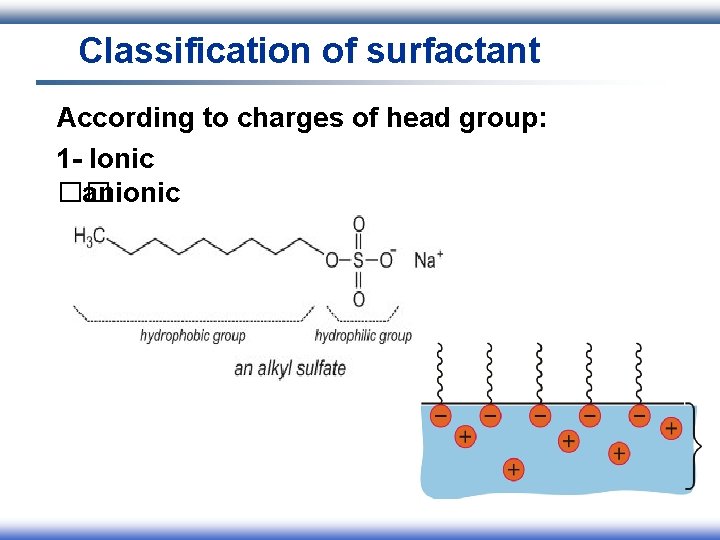
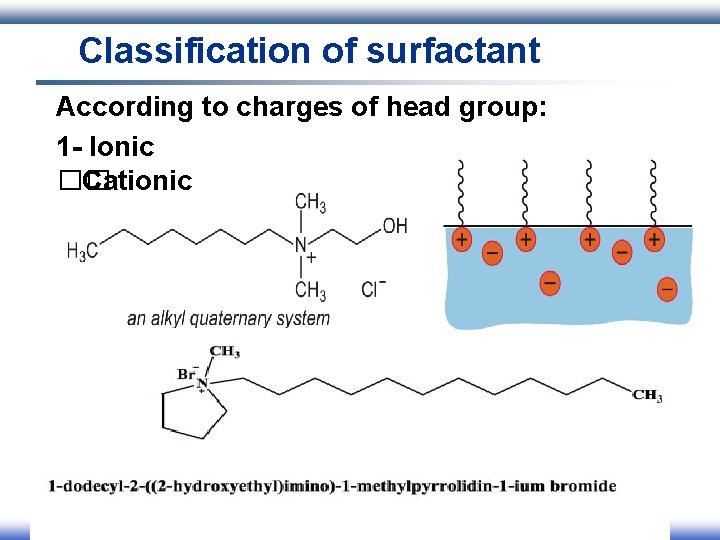
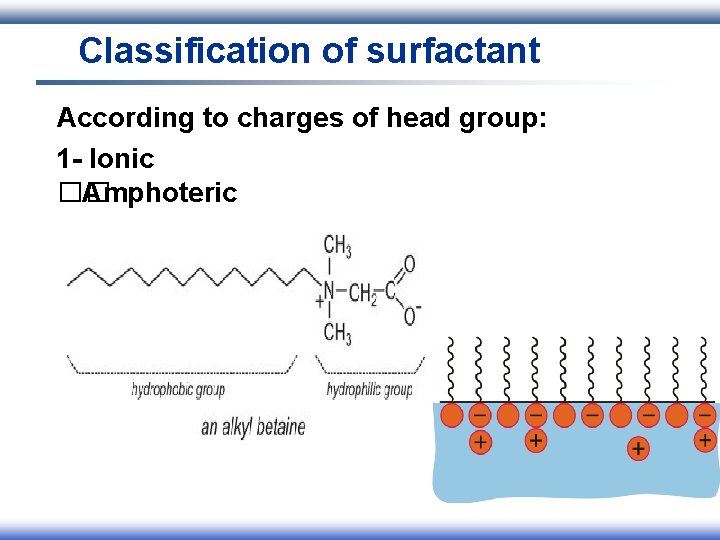
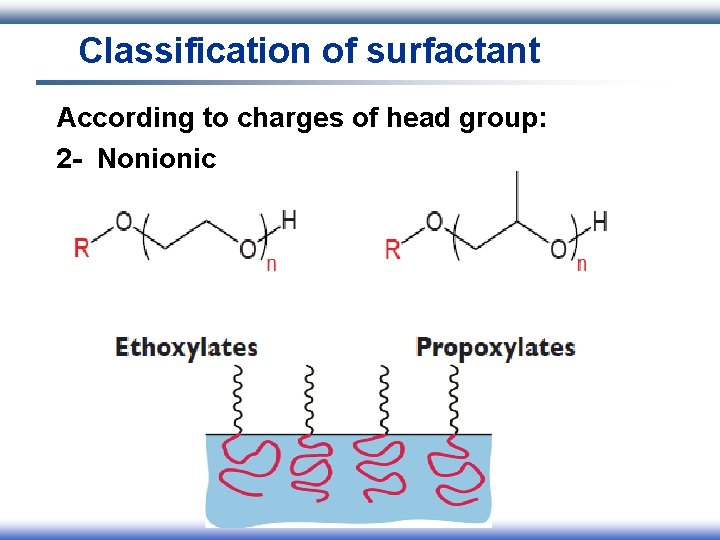
Classification of surfactant According to charges of head group: 1 - Ionic I. Anionic II. Cationic III. Amphoteric 2 - Nonionic

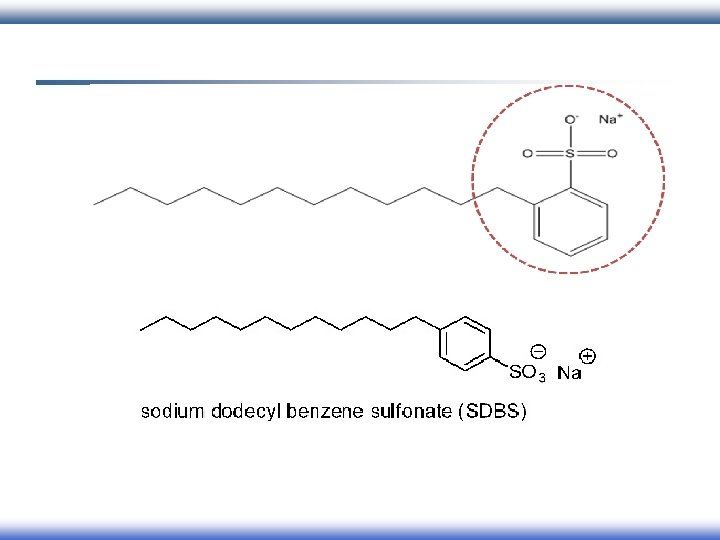
Classification of surfactant According to charges of head group: 1 - Ionic �� anionic

Alkyl ether carboxylate: Alkyl ether sulfate:

Classification of surfactant According to charges of head group: 1 - Ionic �� Cationic

Classification of surfactant According to charges of head group: 1 - Ionic �� Amphoteric

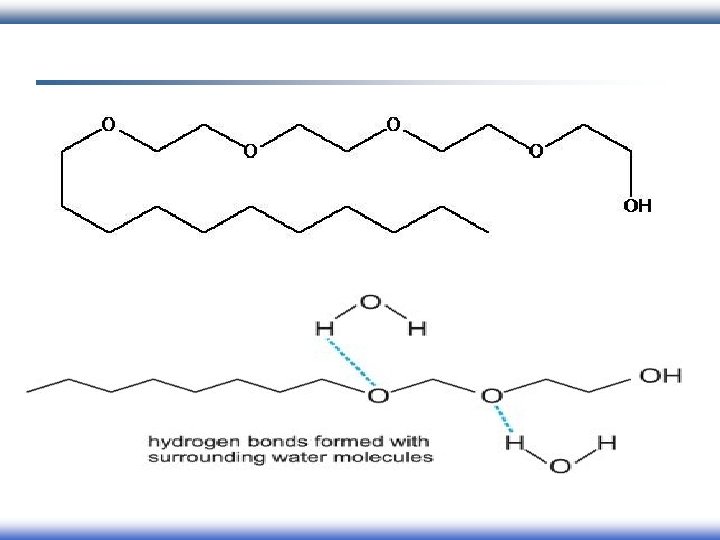
Classification of surfactant According to charges of head group: 2 - Nonionic




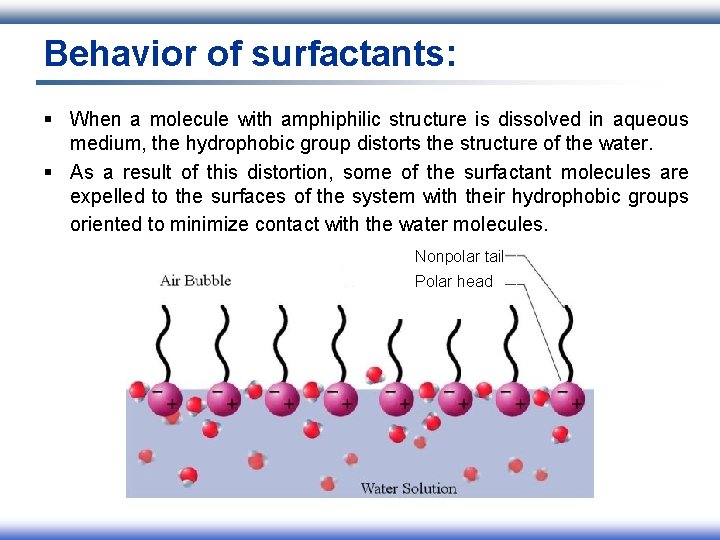
Behavior of surfactants: § When a molecule with amphiphilic structure is dissolved in aqueous medium, the hydrophobic group distorts the structure of the water. § As a result of this distortion, some of the surfactant molecules are expelled to the surfaces of the system with their hydrophobic groups oriented to minimize contact with the water molecules. Nonpolar tail Polar head

Properties of Surfactants

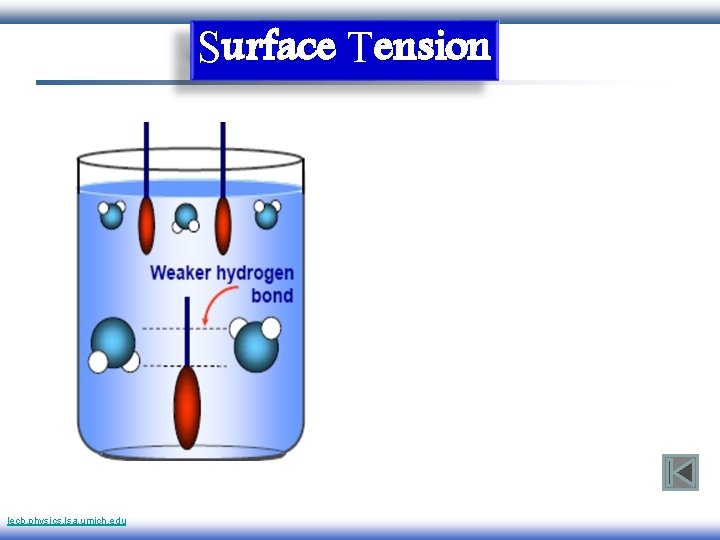
Surface Tension lecb. physics. lsa. umich. edu

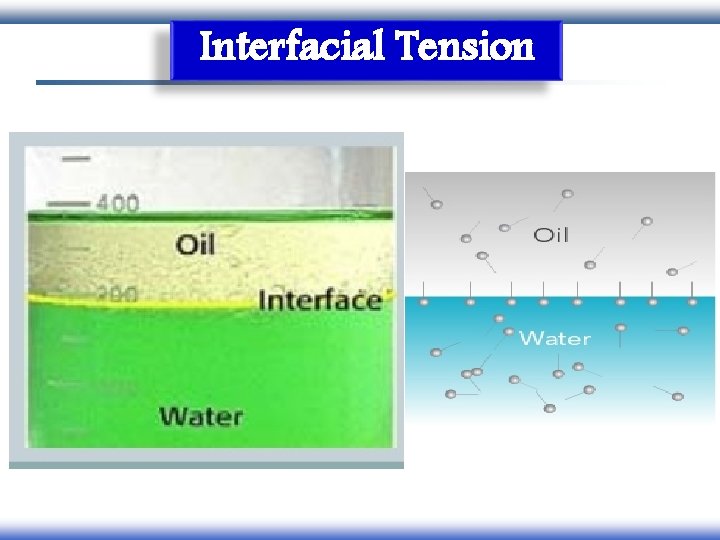
Interfacial Tension

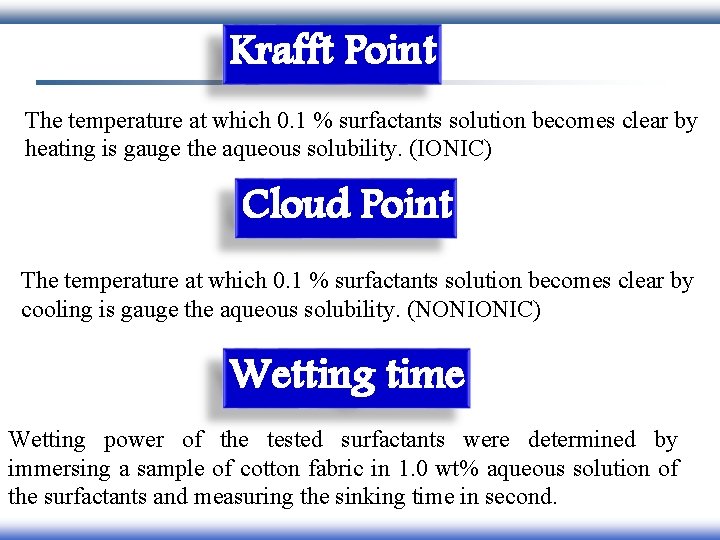
Krafft Point The temperature at which 0. 1 % surfactants solution becomes clear by heating is gauge the aqueous solubility. (IONIC) Cloud Point The temperature at which 0. 1 % surfactants solution becomes clear by cooling is gauge the aqueous solubility. (NONIONIC) Wetting time Wetting power of the tested surfactants were determined by immersing a sample of cotton fabric in 1. 0 wt% aqueous solution of the surfactants and measuring the sinking time in second.

Emulsification power lecb. physics. lsa. umich. edu Examples of Emulsions uses

Foaming Foam consists of gas covered with thin liquid film. Surfactants molecule absorbed to interface between gas and liquid. Foaming agent is a surfactant, which when present in small amounts facilitates the formation of a foam. Food industry Detergent

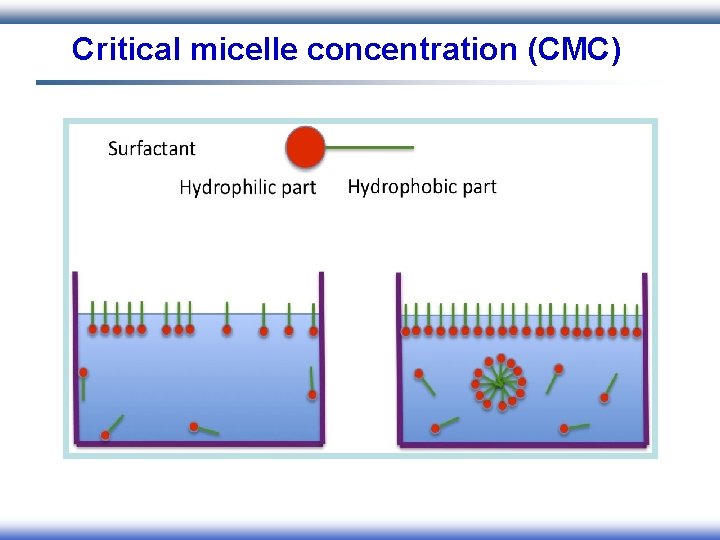
Critical micelle concentration (CMC)

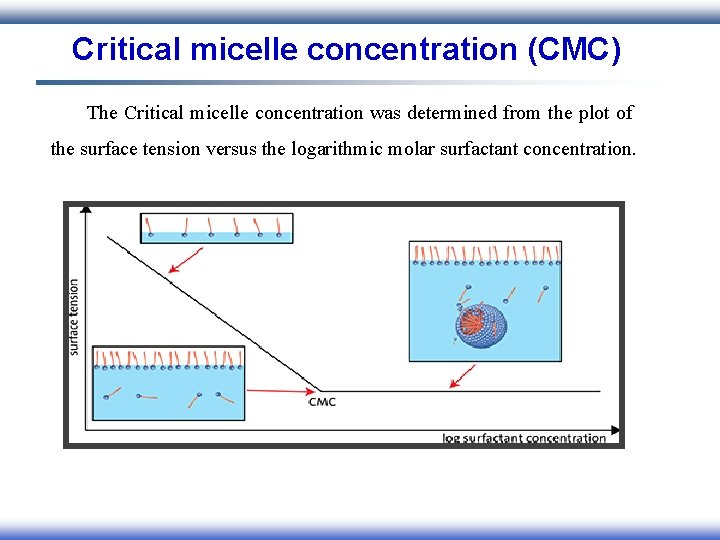
Critical micelle concentration (CMC) The Critical micelle concentration was determined from the plot of the surface tension versus the logarithmic molar surfactant concentration.

Vesicle

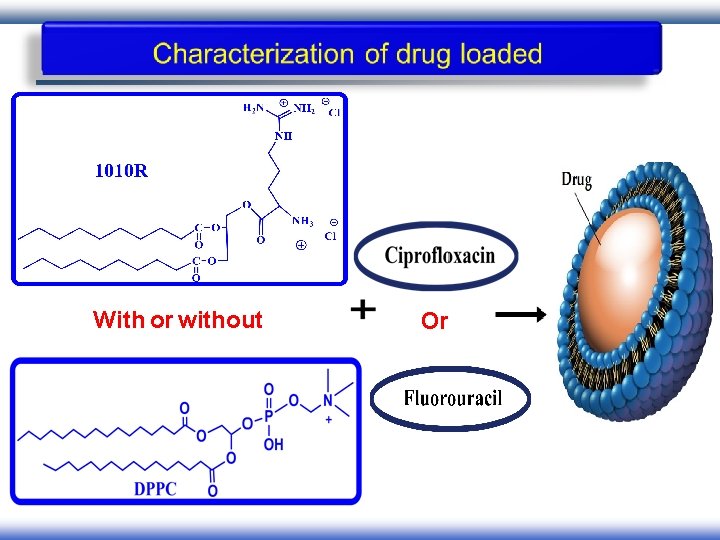
With or without Or

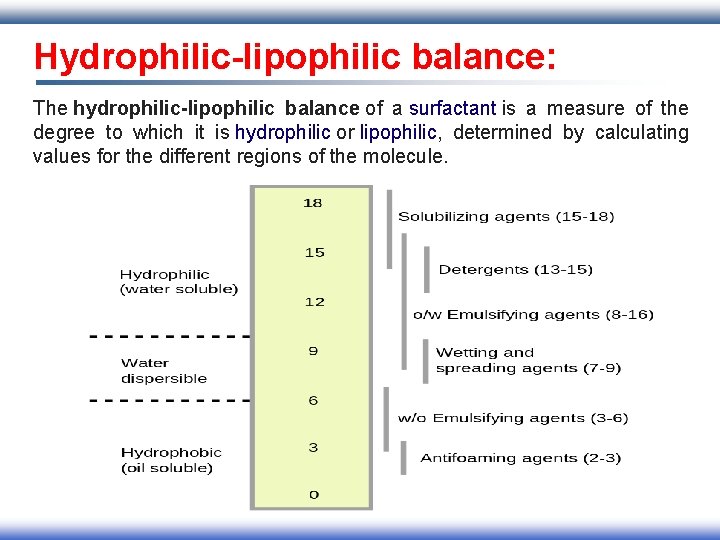
Hydrophilic-lipophilic balance: The hydrophilic-lipophilic balance of a surfactant is a measure of the degree to which it is hydrophilic or lipophilic, determined by calculating values for the different regions of the molecule.

• Kent, J. , Riegel's Handbook of Industrial Chemistry, Van Notrand Reinhold, New York, N. Y. , 1992. • Austin, G. , Shreve's Chemical Process Industries, 5 th ed. , Mc. Graw-Hill Book Company, New York, N. Y. , 1985. • Davidsohn, A. S. and B. Milwidsky, Synthetic Detergents, Longman Scientific and Technical, Burnt Mill, Harlow-England, 1987. • K. Tsujii, “Surface Activity: Principles, Phenomena And Applications”, Academic Press, 1998. • D. J. Mc. Clements, “Food Emulsions: Principles, Practices, and Techniques”, CRC Press, 2 nd ed. , 2005. • K. Robert Lange, “Surfactants: A Practical Handbook”, Hanser, 1999. • J. Goodwin, “Colloids and interfaces with surfactants and polymers”, Wiley 2 nd ed. , 2009. • B. P. Binks, ” Particles as surfactants – similarities and differences”, Current Opinion Colloid Interface Sci. 7 (2002) 21 (review). • N. D. Denkov et al. , “Role of surfactant type and bubble surface mobility in • foam rheology”, Soft Matter 5 (2009) 3389 (review).

http: //www. bu. edu. eg/staff/mohamedaborya 7 -courses/12004/files