So What are Atoms Anyways Are they ants

















- Slides: 17

So What are Atoms Anyways? Are they ants? Are they names for boys?

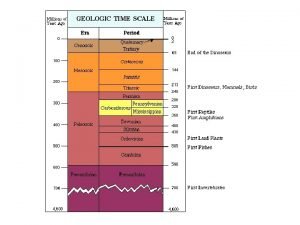
Time to Learn about some OLD dudes. • • • 9/17/2020 Our lesson begins in ancient Greece Some scientists were interested in how small you could make an object I. E. Sugar cane Atomos – means indivisible or not able to be divided The first man to consider atoms was ______. Democritus lived in 440 BCE He said that you could continue to cut an object until it couldn’t be cut anymore. He thought atoms were small, hard particles He thought that atoms were made of the same substance but different sizes Template from www. brainybetty. com 2

Enter Aristotle • He thought Democritus was nuts. • He didn’t think that you could end up with a particle that couldn’t be cut • People respected Aristotle and nobody argued with him about this for a long time. They just accepted he was right and didn’t question him. 9/17/2020 Template from www. brainybetty. com 3

Guess what – Democritus was right! • Matter is made of atoms • Atoms are the smallest parts of _____. • Do you know what pixels are? • Did you know that it would take 105 quadrillion gold atoms to cover the surface of a dollar bill 9/17/2020 Template from www. brainybetty. com 4

Along comes Dalton • In the late 1700’s scientists learned that elements combine in certain ratios. • Dalton, a schoolteacher and chemist wanted to know why • He ran many ____ to draw conclusions about atoms. 9/17/2020 Template from www. brainybetty. com 5

Question Time • 1. The first man to think that atoms couldn’t be divided was a. Dalton b. Thompson c. Democritus d. Aristotle • 2. The first scientist to discover that atoms combine in certain ratios was a. Thompson b. Dalton c. Democritus d. Rutherford Atom Song 9/17/2020 Template from www. brainybetty. com 6

Dalton’s Theory • All substances are made of _______– they can not be created, destroyed or divided • Atoms of the same element are alike, but atoms of different elements are different • Atoms _____ with other atoms to make new substances. 9/17/2020 Template from www. brainybetty. com 7

Guess what – Dalton was wrong too! • In 1897, J. J. Thomson, a British scientist discovered that there were tiny particles inside the atom. • Thomson used a cathode-ray tube to identify negatively charged particles called _____ • Thomson thought that electrons were imbedded throughout the atom like plums in pudding – you might think of it as chocolate chip ice cream • Electrons were originally called _______. 9/17/2020 Template from www. brainybetty. com 8

Let’s throw Rutherford into the mix! • In 1909, Ernest Rutherford decided to test Thomson’s theory • Rutherford aimed a beam of small positively charged particles at a thin sheet of gold • Rutherford put a special coating behind the foil • The coating ____ when the positive particles hit it. • This allowed Rutherford to see where the particles went after they hit the foil 9/17/2020 Template from www. brainybetty. com 9

What did Rutherford find out? • Some particles went straight through • Some particles were deflected to one side • Some particles bounced straight back 9/17/2020 Template from www. brainybetty. com 10

Question Time • 1. The first scientist to find particles inside atoms was a. Dalton b. Rutherford c. Thompson d. Bohr • 2. Rutherford learned that the particles in the atoms did all of the following except a. stayed put b. went straight through c. were deflected d. bounced straight back 9/17/2020 Template from www. brainybetty. com 11

So Where are the Electrons? • In 1911, Rutherford revised the atomic theory • Rutherford proposed that in the center of the atom is a tiny dense positively charged part called the ____ • Rutherford said that any positive particle coming close to the nucleus would be repelled because like charges do that • Rutherford calculated that the nucleus was 100, 000 times smaller than the diameter of a gold atom. 9/17/2020 Template from www. brainybetty. com 12

So Who was Bohr? • In 1913, Neils Bohr – who worked with Rutherford studied the way atoms react to light • He said electrons travel in _______ or _____ around the nucleus • In Bohr’s model there was no pathway • between the levels 9/17/2020 Template from www. brainybetty. com 13

Finally, the Modern Atomic Theory • Erwin Schrodinger and Werner Heisenberg explained the nature of electrons in the atom • Exact path of electrons can’t be predicted • Inside the atom there areas where electrons are likely to be – these regions are called _________ 9/17/2020 Template from www. brainybetty. com 14

Answer Me This • Why do you think Dalton’s atomic theory is in Science books? • What discovery demonstrated that atoms are mostly empty space? • How does the location of the electron compare in Bohr’s theory compared to the modern theory? 9/17/2020 Template from www. brainybetty. com 15

Question Time • 1. Rutherford said there was a dense part of the atom called the a. neutron b. proton c. nucleus d. electron • 2. Bohr thought electrons traveled a. inside the atom b. in levels outside the atoms c. at random outside the nucleus • 3. In the atomic theory we use today we know that electrons travel in a. levels b. in electron clouds c. orbitals 9/17/2020 Template from www. brainybetty. com 16

Time to Summarize • Democritus thought that matter is composed of atoms • Dalton based his theory on observations of how elements combine • Thomson discovered electrons in atoms • Rutherford discovered that atoms are mostly empty space with a dense, positive nucleus • Bohr proposed that electrons are located in levels at certain distances from the nucleus • The electron cloud model represents the current atomic theory. 9/17/2020 Template from www. brainybetty. com 17
 Mikael ferm
Mikael ferm Regents periodic table
Regents periodic table As atoms bond with each other they
As atoms bond with each other they When atoms combine they form
When atoms combine they form Symbiotic relationship
Symbiotic relationship Ant anatomy
Ant anatomy Ants tammepuu
Ants tammepuu Ants philosophy
Ants philosophy Roland matt
Roland matt Njourneyn.1
Njourneyn.1 Ant larvae
Ant larvae The ant and the grasshopper venn diagram
The ant and the grasshopper venn diagram Why do ants live underground
Why do ants live underground Ants
Ants What do we learn from ants
What do we learn from ants Lubo ants
Lubo ants Ants torim
Ants torim Ants
Ants