Snowman Challenge Balancing Equations Translating Chemical Equations Objective

Snowman Challenge (Balancing Equations) Translating Chemical Equations
Objective: ◦ Today I will be able to: Apply the law of conservation of matter to balancing chemical equations Translate chemical equations from words to symbols and symbols to words Evaluation/Assessment: ◦ Informal Assessment – listening to student interactions as they complete the snowman challenge and the translating equations practice. ◦ Formal Assessment – analyzing student responses to the exit ticket and translating equations practice Common Core Connection ◦ Make sense of problem and persevere in solving them ◦ Reason abstractly and quantitatively ◦ Look for and express regularity in repeated reasoning

Lesson Sequence Evaluate: Warm – Up Evaluate: Review Homework Elaborate: Snowman Challenge, Balancing Equations Explain: Translating and Properties of Equations Elaborate: Translating Equations Practice Evaluate: Exit Ticket
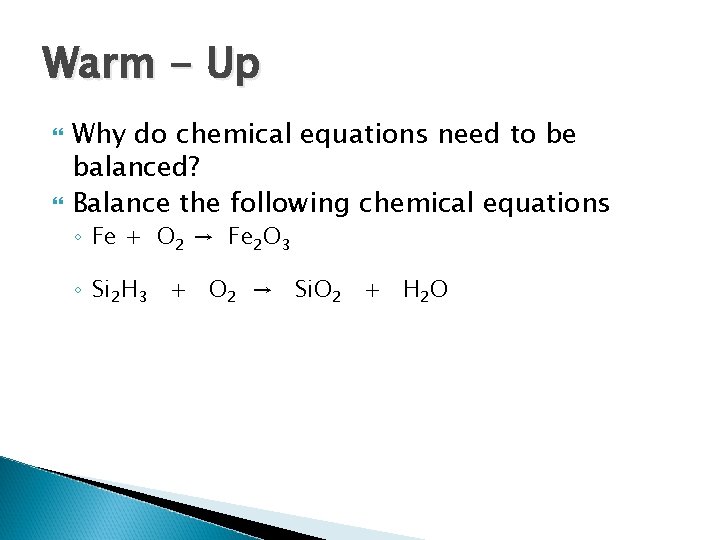
Warm - Up Why do chemical equations need to be balanced? Balance the following chemical equations ◦ Fe + O 2 → Fe 2 O 3 ◦ Si 2 H 3 + O 2 → Si. O 2 + H 2 O

Objective Today I will be able to: ◦ Apply the law of conservation of matter to balancing chemical equations ◦ Translate chemical equations from words to symbols and symbols to words

Homework Finish Translating Equations Practice

Agenda Warm – Up Review Homework Snowman Challenge Translating Equations Notes Translating Equations Practice Exit Ticket

Review Homework We will review selected problems as a class and then turn in the Balancing Chemical Equations Practice 1

Snowman Challenge

Snowman Challenge Rules Work in groups of 3 (at your row) to balance equations When you finish balancing your equations, bring your paper to Ms. Ose to check your answers The first team to have all the correct answers wins a prize Do not begin until Ms. Ose says GO!
Translating Chemical Equations and Properties of Chemical Reactions Notes

What is a chemical reaction? Process of one or more reactants converting to form products with different properties.

Ways to Represent Chemical Equations Using words ◦ Hydrogen (gas) + Oxygen (gas) water (liquid) Using symbols ◦ H 2(g) + O 2(g) 2 H 2 O(l) Symbols are a shorthand way to represent an equation
Evidence of Chemical Reactions Production of a gas Production of a solid ◦ Precipitate – a solid formed as the product of a chemical reaction A color change Temperature change

Translating Chemical Equations Practice Complete the practice at your desk and whatever you do not finish is homework

Exit Ticket For the following chemical reaction: ◦ Solid mercury (II) oxide breaks down when heated, forming the elements mercury and oxygen. Write the word equation Write the balanced formula equation (symbols) ◦ Make sure you include the correct state!
- Slides: 16