Smog When smoke and fog combine Two types

Smog When “smoke” and “fog” combine Two types • Photochemical smog • Coal induced smog

The Birth of Smog First began appearing inn the early 19 th century in London due to the conditions of coal smoke and fog experienced by the city -This smog is known as industrial, or reducing, smog -Caused by the combustion of sulfur found in the coal used for heating, manufacturing, and the production of electricity -Consists of water droplets, sulfur dioxide and trioxide, soot, ash, and sulfuric acid

Industrial coal burning Smog Found in coal burning cities which experience cold, wet winters, and thermal inversion which traps cool, stagnant air close to the earth’s surface However, due to increased pollution controls, bans on coal use as a domestic fuel, and the use of alkaline scrubbers (which remove Sox, severe occurrences of industrial smog are diminishing

Industrial Smog (Reducing) • Source: Pollution from the burning of coal and oil that contains sulfur • Consists mainly of: Sulfur Dioxide, Sulfur Trioxide, soot and ash (particulate matter) and sulfuric acid • It can cause breathing difficulties in humans, plus acid rain damage to plants, aquatic systems, and metal or stone objects • London and Chicago have problems with industrial smog. • Methods of reducing this smog: Alkaline Scrubbers reduce SO 2 and SO 3 levels; electrostatic precipitators reduce particulates.

Photochemical Smog First observed in the 1940’s in Los Angeles Secondary air pollutant Unlike industrial smog, the paramount cause of photochemical smog is a combination of automobile pollution and ultraviolet radiation from the sun Comprised of hundreds of substances Mainly resultant of Nitrogen oxide reaction with ultravoilet light Each substance is formed by free radical reactions facilitated by ultraviolet radiation - A free radical is a highly reactive entity with one or more unpaired electrons

Main Components of Photochemical Smog Noxious mixture of highly reactive and oxidizing air pollutants including: • Oxides of Nitrogen (NOx) • Volatile organic compounds • Troposphere Ozone • Peroxyacetyl Nitrates (PAN)
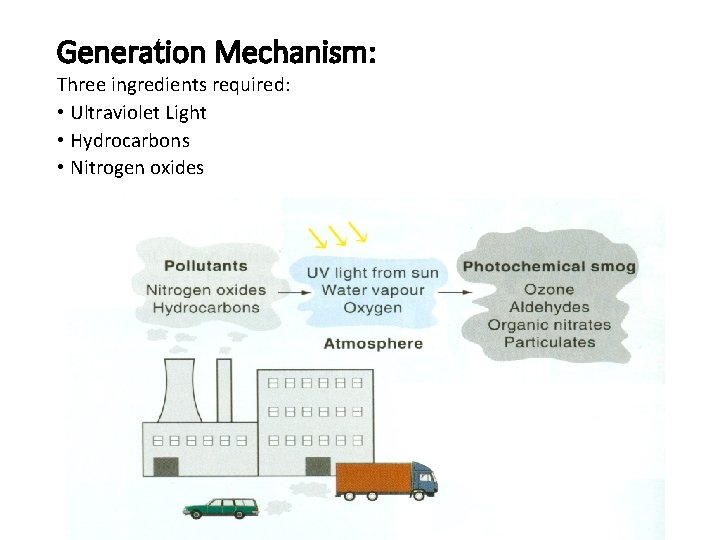
Generation Mechanism: Three ingredients required: • Ultraviolet Light • Hydrocarbons • Nitrogen oxides
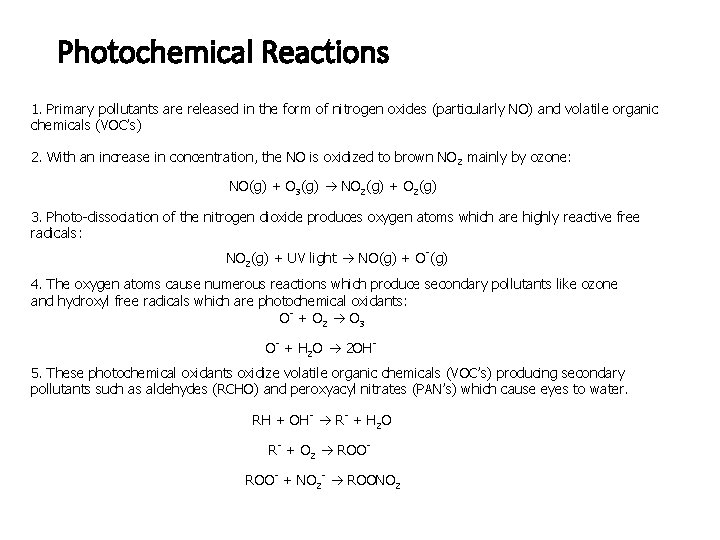
Photochemical Reactions 1. Primary pollutants are released in the form of nitrogen oxides (particularly NO) and volatile organic chemicals (VOC’s) 2. With an increase in concentration, the NO is oxidized to brown NO 2 mainly by ozone: NO(g) + O 3(g) NO 2(g) + O 2(g) 3. Photo-dissociation of the nitrogen dioxide produces oxygen atoms which are highly reactive free radicals: NO 2(g) + UV light NO(g) + O-(g) 4. The oxygen atoms cause numerous reactions which produce secondary pollutants like ozone and hydroxyl free radicals which are photochemical oxidants: O- + O 2 O 3 O- + H 2 O 2 OH 5. These photochemical oxidants oxidize volatile organic chemicals (VOC’s) producing secondary pollutants such as aldehydes (RCHO) and peroxyacyl nitrates (PAN’s) which cause eyes to water. RH + OH- R- + H 2 O R- + O 2 ROOROO- + NO 2 - ROONO 2
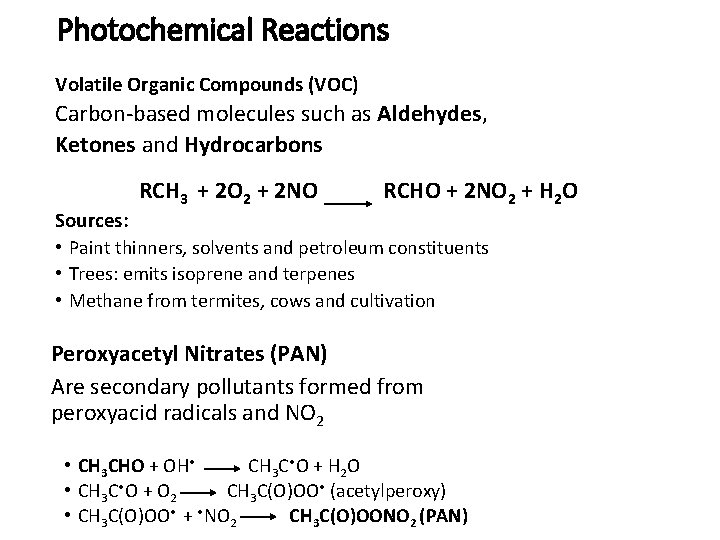
Photochemical Reactions Volatile Organic Compounds (VOC) Carbon-based molecules such as Aldehydes, Ketones and Hydrocarbons RCH 3 + 2 O 2 + 2 NO RCHO + 2 NO 2 + H 2 O Sources: • Paint thinners, solvents and petroleum constituents • Trees: emits isoprene and terpenes • Methane from termites, cows and cultivation Peroxyacetyl Nitrates (PAN) Are secondary pollutants formed from peroxyacid radicals and NO 2 • CH 3 CHO + OH • CH 3 C • O + H 2 O • CH 3 C • O + O 2 CH 3 C(O)OO • (acetylperoxy) • CH 3 C(O)OO • + • NO 2 CH 3 C(O)OONO 2 (PAN)

Effects on human health: • Ozone • • • Cause acute respiratory problems Aggravate asthma Cause temporary decreases in lung function in healthy adults Lead to hospital admissions and emergency room visits Impair the body's immune system • Peroxyacetylnitrate (PANs) • Respiratory and eye irritants • Mutagenic- causing skin cancer • Volatile organic compounds (VOCs) • • Global warming- Methane Carcinogenic- benzene Form Ozone Eye, nose, and throat irritation; headaches; damage to liver, kidney, and central nervous system. Some organics can cause cancer in animals; some are suspected or known to cause cancer in humans
- Slides: 10