Smog Smoke and fog London Smog Industrial smog











- Slides: 24

Smog • Smoke and fog • London Smog / Industrial smog / reducing smog • Caused by the combustion of sulfur containing coal • Water droplets, SO 2, SO 3, soot, fly ash and sulfuric acid

Reducing Smog • Chicago, Beijing • Cold, wet winters • Thermal inversion traps cold air close to earth surface • Decline in reducing smog • Use of SNG, alkaline scrubber

London Smog

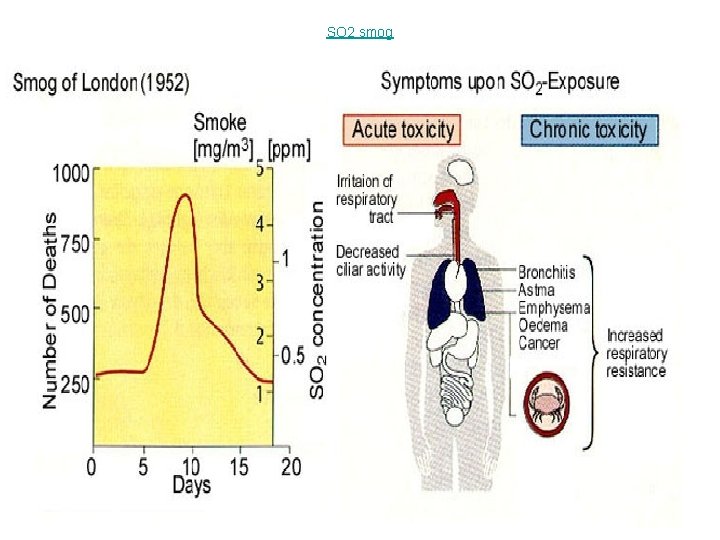
London Smog : SO 2 • water droplets in the air condenses on smoke particles produced by the burning of coal, forming tiny smog droplets. -sulfur dioxide -Sulfur dioxide is a gas that attacks the lungs and makes breathing difficult

SO 2 smog

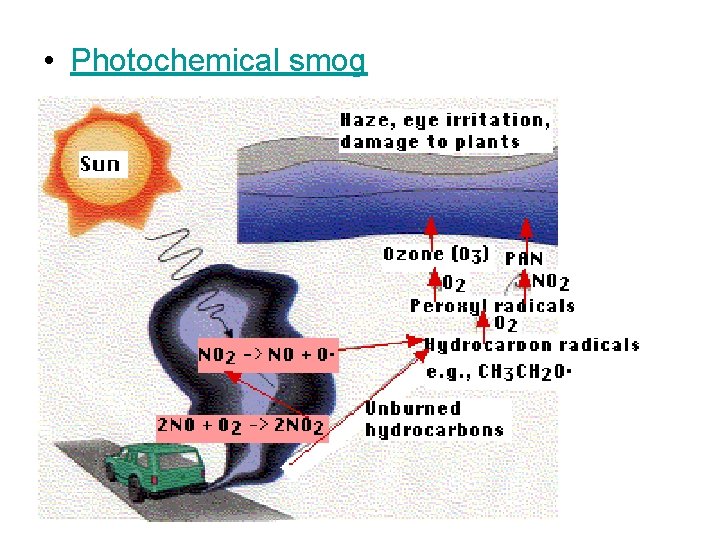
Photochemical smog Oxidizing smog

• Photochemical smog

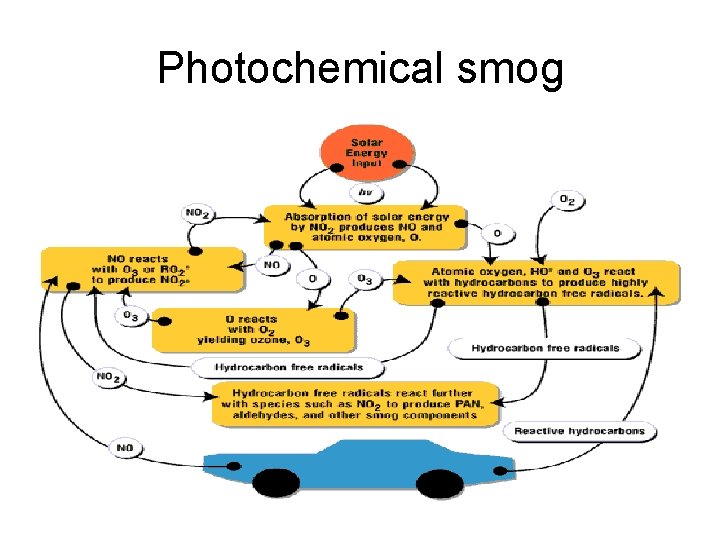
Photochemical smog



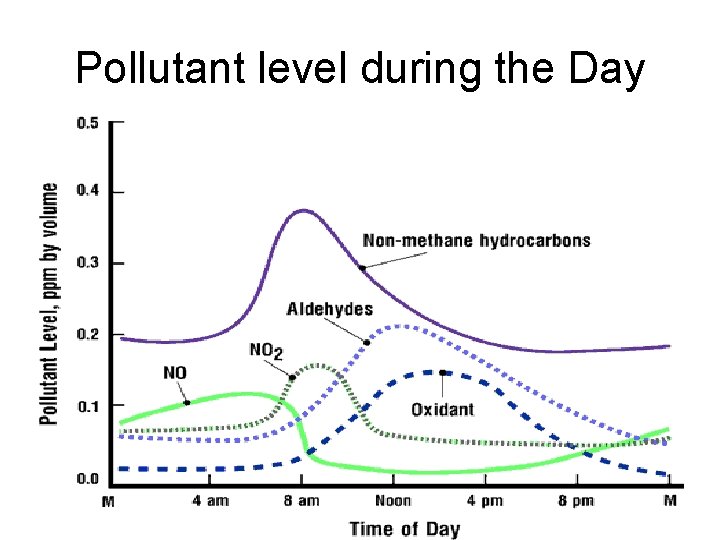
Photochemical smog • clear, sunny skies • warm temperatures • stable air conditions under a stationary high pressure system • onshore sea-breeze

Pollutant level during the Day

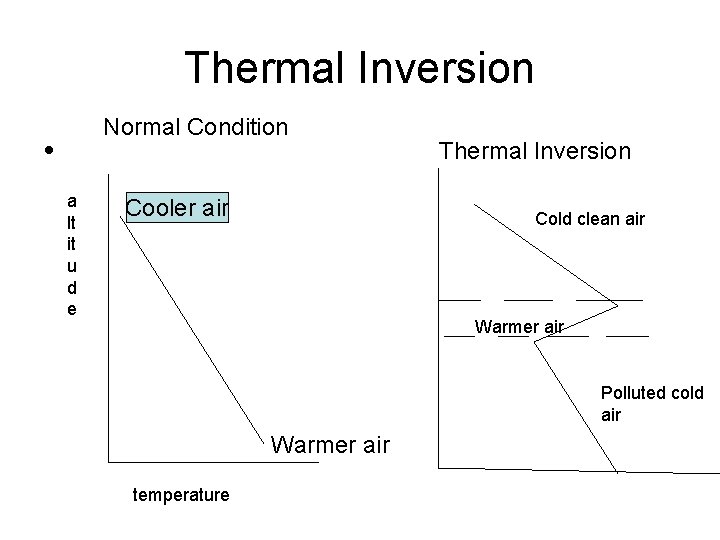
Thermal Inversion

E 10 Thermal Inversion Effects on Air Quality

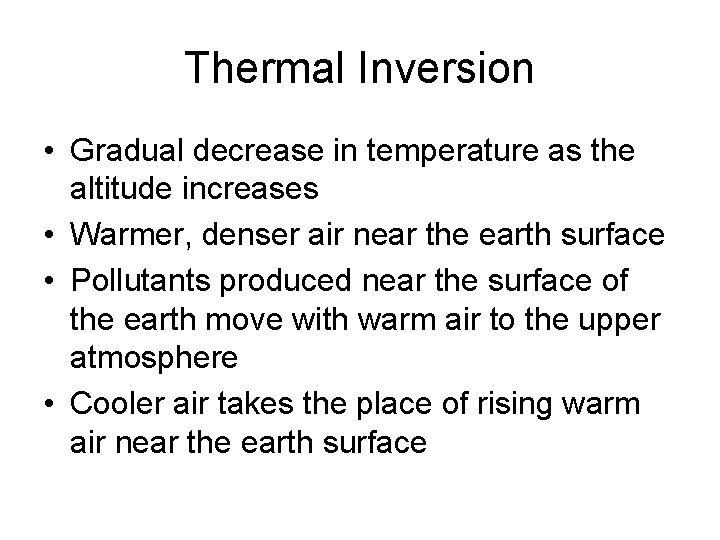
Thermal Inversion • Gradual decrease in temperature as the altitude increases • Warmer, denser air near the earth surface • Pollutants produced near the surface of the earth move with warm air to the upper atmosphere • Cooler air takes the place of rising warm air near the earth surface

Thermal Inversion • Air currents allow the pollutants to be dispersed • Abnormal temperature arrangement of air masses where a layer of warmer air is trapped between the two layers of cold air • Wind gives horizontal movement of air • Mountains hinders the dispersion • Flow of cold air creates cool layer

Thermal Inversion • No wind, cold temperatures, , mountains • Warmer air acts as lid

Thermal Inversion • a lt it u d e Normal Condition Cooler air Thermal Inversion Cold clean air Warmer air Polluted cold air Warmer air temperature

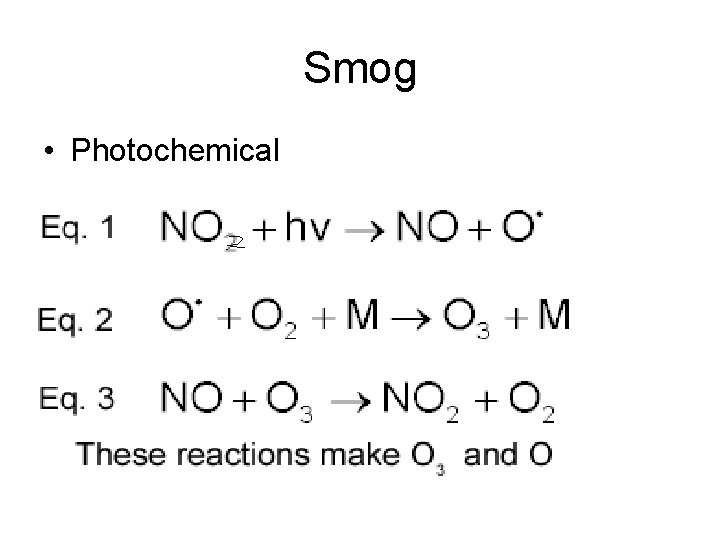
Smog • Photochemical

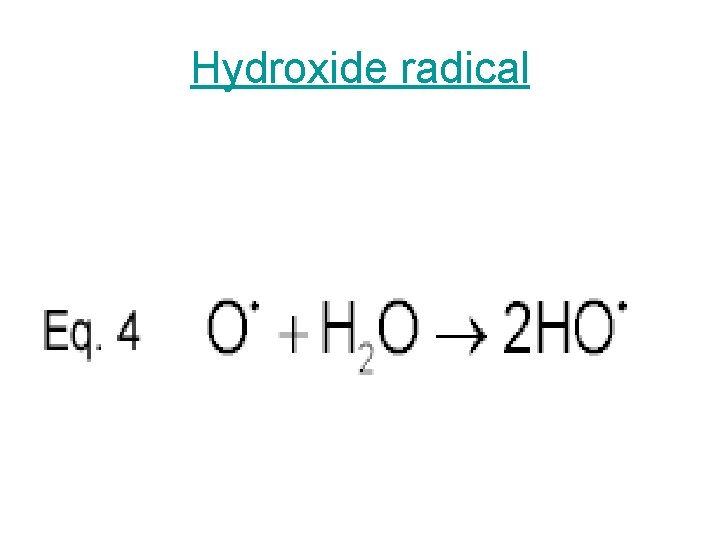
Hydroxide radical

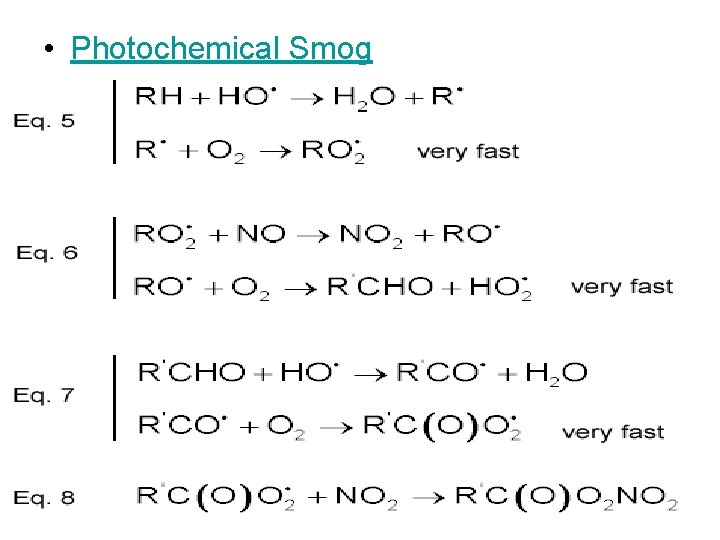
• Photochemical Smog

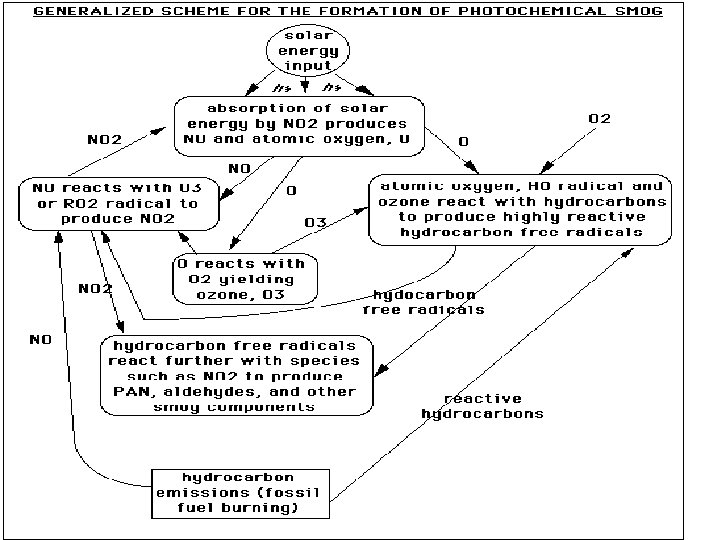
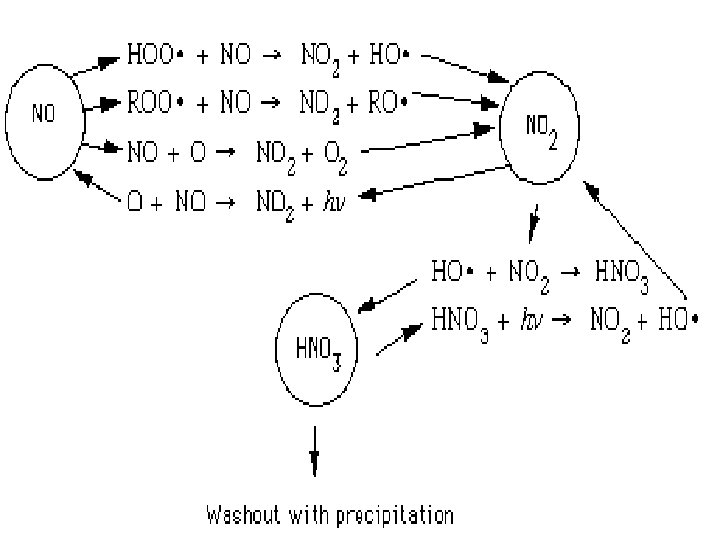
In summary, this is what happens in photochemical smog formation • 1) Nitrogen oxides generate oxygen atoms • 2) Oxygen atoms form hydroxyl radicals • 3) Hydroxyl radicals generate hydrocarbon radicals • 4) Hydrocarbon radicals form hydrocarbon peroxides • 5) Hydrocarbon peroxides form aldehydes • 6) Aldehydes form aldehyde peroxides • 7) Aldehyde peroxides form peroxyacylnitrates

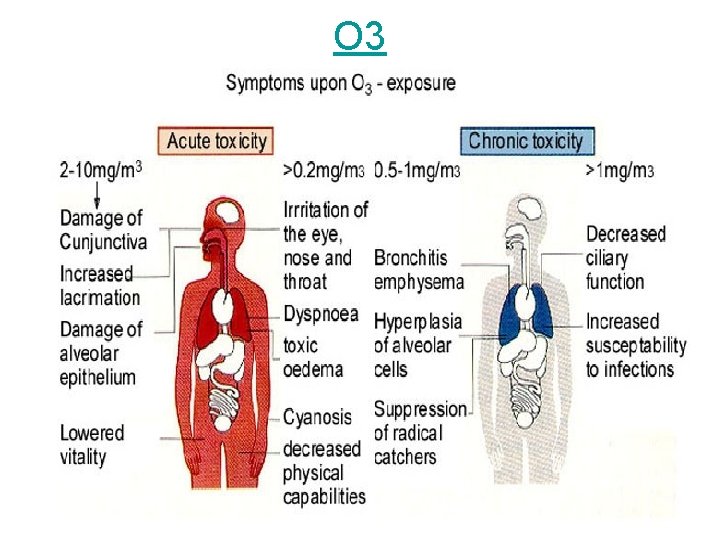
O 3

Ethanol