SMFM Consult Series 38 Hepatitis B in pregnancy
SMFM Consult Series # 38 Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission Society of Maternal Fetal Medicine with the assistance of Jodie Dionne-Odom, MD; Alan T. N. Tita, MD, Ph. D; Neil S. Silverman, MD Published in AJOG/ January 2016

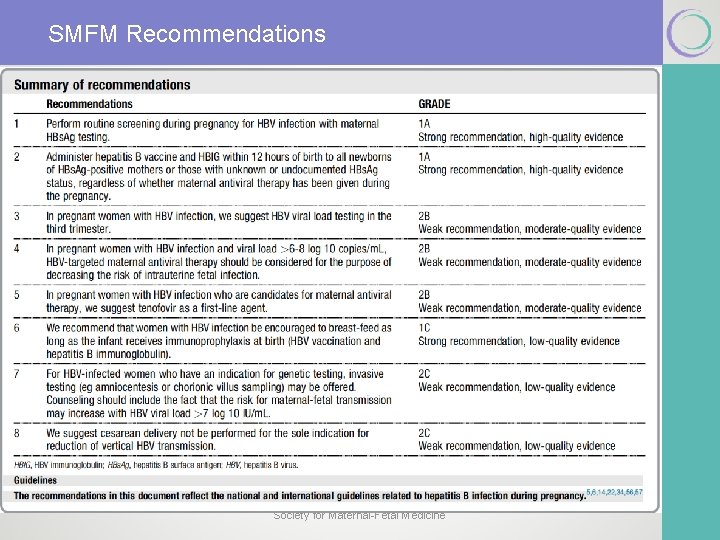
What risks and potential impact does hepatitis B infection present during pregnancy? § Chronic HBV infection is the major source of hepatocellular carcinoma. § In the United States, vertical transmission is responsible for up to 50% of HBV infection worldwide. § In pregnancy, prevalence 0. 7 -0. 9% for chronic HBV § Universal screening for HBV in pregnancy at the first prenatal visit. § Cirrhosis due to chronic HBV may be associated with increased maternal and perinatal death, gestational hypertension, abruption, pretermbirth, and fetal growth restriction.
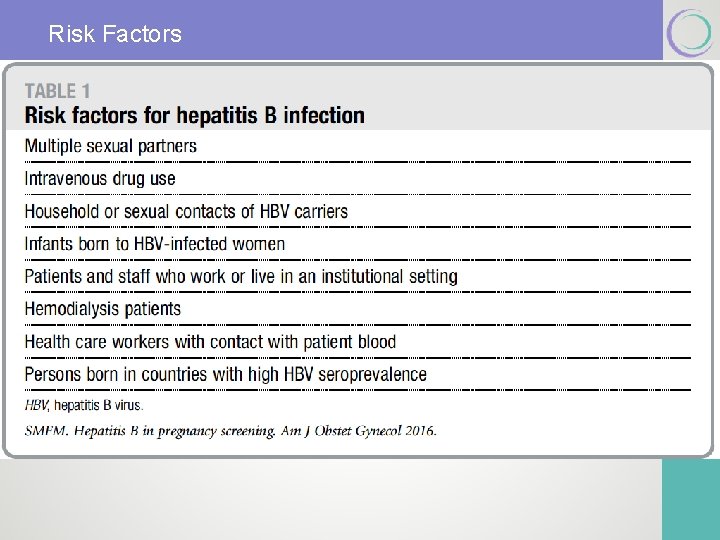
Risk Factors
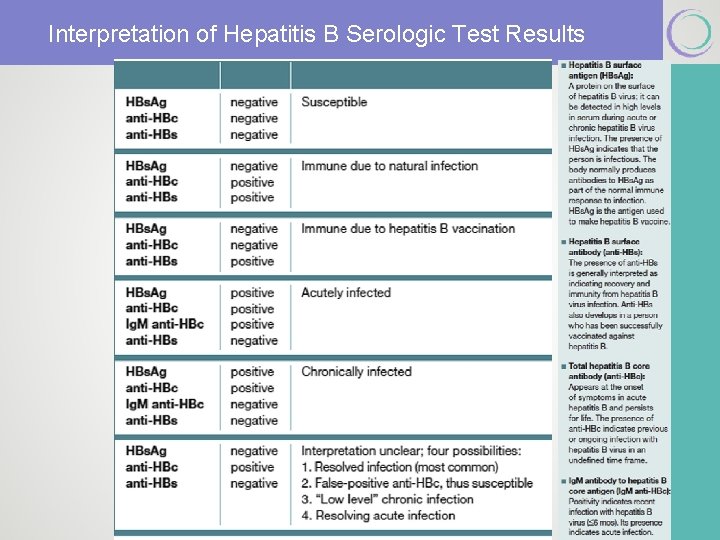
Interpretation of Hepatitis B Serologic Test Results

How are HBV-infected pregnant women identified and what have been traditional approaches to their pregnancies? § The diagnosis of the chronic carrier state is confirmed with persistence of HBs. Ag and the absence of hepatitis B surface antibody (HBs. Ab). § HBs. Ag and HBs. Ab do not coexist. § Start with maternal HBs. Ag. § HBV core ab is produced secondary to infection (never from immunization). § Pregnancy is not a C/I for HBV vaccine. Society for Maternal-Fetal Medicine

How are HBV-infected pregnant women identified and what have been traditional approaches to their pregnancies? § Perinatal Transmission § Most common risk include infected vaginal blood and secretions at the time of delivery. § Invasive procedures during (FSE, amnio, OVD. . ) theoretically increase risk of transmission § Higher risk of transmission if high viral load (> 7 log 10 IU/m. L) § CD not recommended § Breast feeding not C/I as long as the infant receives immunoprophylaxis at birth (HBV vaccination and hepatitis B immunoglobulin) Society for Maternal-Fetal Medicine

The traditional approach to preventing neonatal HBV infection: Neonatal Immunization § Combination of HBV immunoglobulin (passive) & HBV vaccine (active) within 12 hours of delivery. § Long-term immunity in 85 -95% of cases. § Given to exposed infants and those born to mothers with unknown or undocumented HBs. Ag status. § Completion of the full 3 -dose HBV vaccine series (all children).
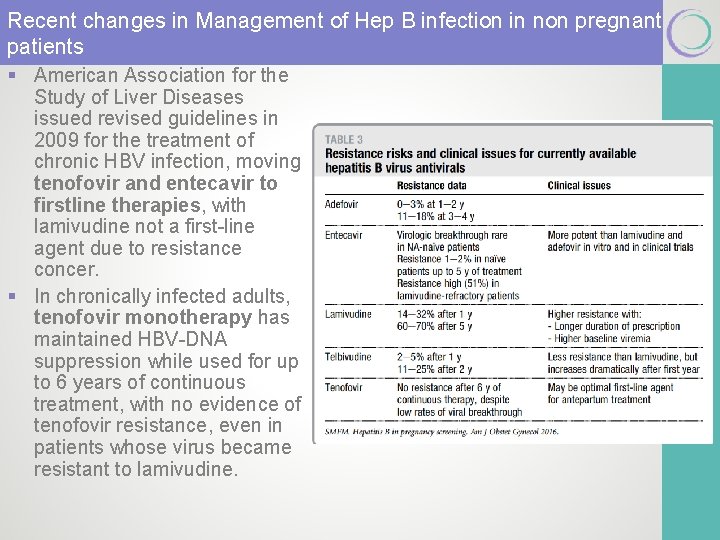
Recent changes in Management of Hep B infection in non pregnant patients § American Association for the Study of Liver Diseases issued revised guidelines in 2009 for the treatment of chronic HBV infection, moving tenofovir and entecavir to firstline therapies, with lamivudine not a first-line agent due to resistance concer. § In chronically infected adults, tenofovir monotherapy has maintained HBV-DNA suppression while used for up to 6 years of continuous treatment, with no evidence of tenofovir resistance, even in patients whose virus became resistant to lamivudine.

New Approach in Management of Hep B During Pregnancy § HBV viral load in the third trimester (28 -32 weeks) § If> 6 -8 log 10 copies /m. L – antiretroviral Rx to decrease risk of intrauterine transmission § To convert viral load from IU/m. L to copies /m. L multiply x 5. 6 § Tenofovir (first line) 300 mg/day till delivery § Lamivudine (high rate of resistance) if used as a single agent § Indications for antiretroviral § Copies > 106 -8 § Pos HBe. Ag § The use of lamivudine and tenofovir in the postpartum period is not currently recommended solely for HBV prevention until additional data are available.

Care of Pregnant Chronic HBV Carriers § Family and household members should be evaluated for HBV status and referred for vaccination if found to be uninfected and nonimmune. § The pregnant woman herself should also be assessed for immunity status for hepatitis A and offered vaccination if not immune. § The woman should also be counseled regarding exposures to potentially hepatotoxic medications (acetaminophen, and to avoid the use of alcohol even when not pregnant. § Positive HBs. Ag Baseline LFTs and a baseline quantitative HBVDNA level. § If DNA negative , repeat third trimester (optimal time to start antiretroviral Rx in women with high viral load. )
SMFM Recommendations Society for Maternal-Fetal Medicine

Disclaimer § The practice of medicine continues to evolve, and individual circumstances will vary. This opinion reflects information available at the time of its submission for publication and is neither designed nor intended to establish an exclusive standard of perinatal care. This presentation is not expected to reflect the opinions of all members of the Society for Maternal-Fetal Medicine. § These slides are for personal, non-commercial and educational use only

Disclosures § All authors and Committee members have filed conflict of interest disclosure delineating personal, professional, and/or business interests that might be perceived as a real or potential conflict of interest in relation to this publication. Any conflicts have been resolved through a process approved by the Executive Board. The Society for Maternal-Fetal Medicine has neither solicited nor accepted any commercial involvement in the development of the content of this publication.
- Slides: 13