Small White Matter Lesion Detection in Cerebral Small

Small White Matter Lesion Detection in Cerebral Small Vessel Disease Mohsen Ghafooriana, b, Nico Karssemeijera, Inge van Udenc, Frank-Erik de Leeuwc, Tom Heskesb, Elena Marchiorib and Bram Platela Diagnostic Image Analysis Group, Radiology Department, Radboudumc, Nijmegen, the Netherlands Institute for Computing and Information Sciences, Radboud University, Nijmegen, The Netherlands c Donders Institute for Brain, Cognition and Behaviour, Department of Neurology, Radboudumc, Nijmegen, The Netherlands a b

Small Vessel Disease • The term cerebral small vessel disease (SVD) refers to a group of pathological processes with various causes that affect the small arteries of the brain • Prevalent in elderly people • Symptoms are disturbances in: • Cognition • Motor • Mood * A. Charidimou et al. , Front. Neur. 2012
Small Vessel Disease • Only in some cases SVD leads to • Cognitive impairment • Motor impairment • Dementia • Parkinsonism • White matter lesions: a common observation on SVD patients • To this end studies are being conducted to investigate cognitive and motor performance in relation to WML locational distribution, total load and progress. • Including the RUNDMC* study. * van Norden et al. BMC Neurology 2011
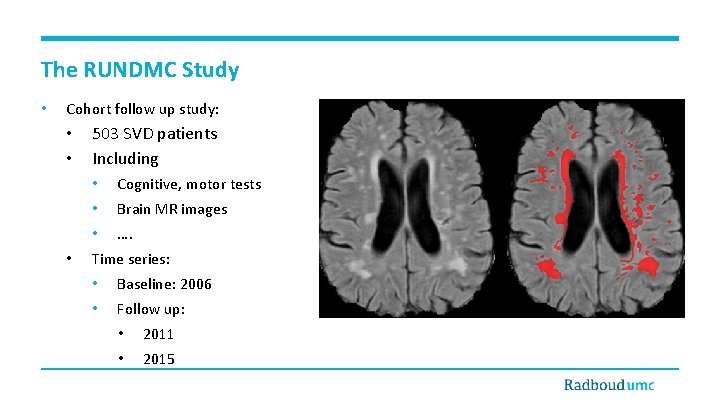
The RUNDMC Study • Cohort follow up study: • • • 503 SVD patients Including • Cognitive, motor tests • Brain MR images • …. Time series: • Baseline: 2006 • Follow up: • 2011 • 2015
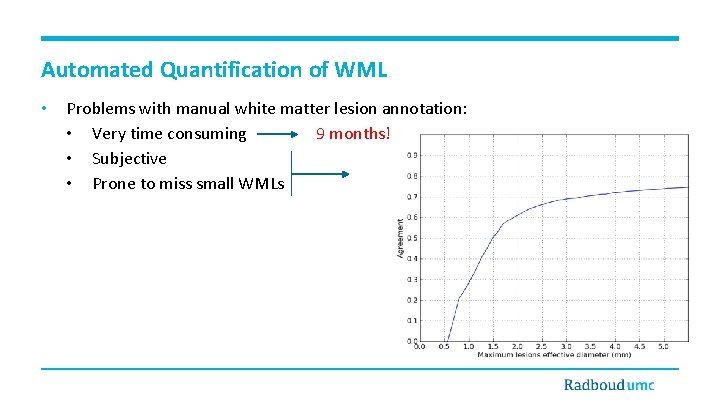
Automated Quantification of WML • Problems with manual white matter lesion annotation: • Very time consuming 9 months! • Subjective • Prone to miss small WMLs
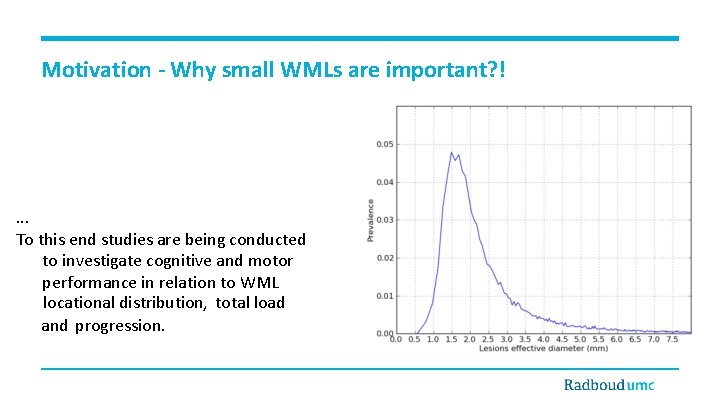
Motivation - Why small WMLs are important? ! . . . To this end studies are being conducted to investigate cognitive and motor performance in relation to WML locational distribution, total load and progression.

Purpose • Development of a voxel based CAD system to detect small white matter lesions as accurate as possible.
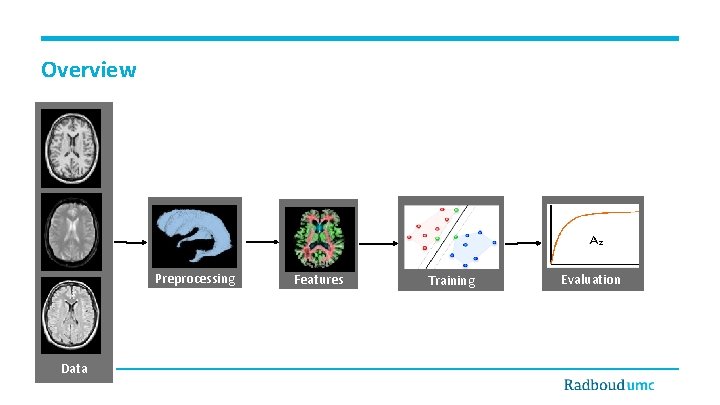
Overview Preprocessing Data Features Training Evaluation
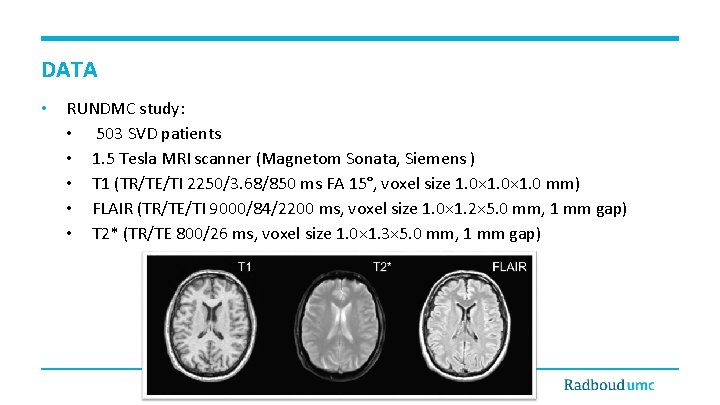
DATA • RUNDMC study: • 503 SVD patients • 1. 5 Tesla MRI scanner (Magnetom Sonata, Siemens ) • T 1 (TR/TE/TI 2250/3. 68/850 ms FA 15°, voxel size 1. 0× 1. 0 mm) • FLAIR (TR/TE/TI 9000/84/2200 ms, voxel size 1. 0× 1. 2× 5. 0 mm, 1 mm gap) • T 2* (TR/TE 800/26 ms, voxel size 1. 0× 1. 3× 5. 0 mm, 1 mm gap)
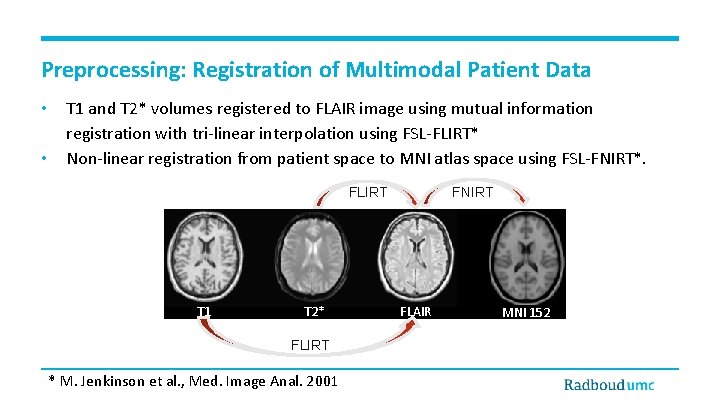
Preprocessing: Registration of Multimodal Patient Data • • T 1 and T 2* volumes registered to FLAIR image using mutual information registration with tri-linear interpolation using FSL-FLIRT* Non-linear registration from patient space to MNI atlas space using FSL-FNIRT*. FLIRT T 1 T 2* FLIRT * M. Jenkinson et al. , Med. Image Anal. 2001 FNIRT FLAIR MNI 152
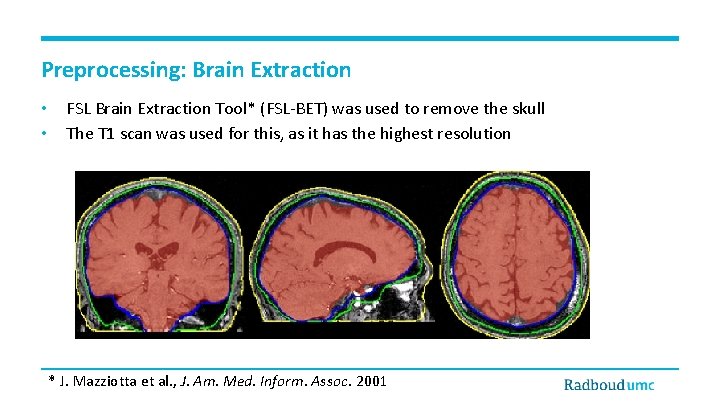
Preprocessing: Brain Extraction • • FSL Brain Extraction Tool* (FSL-BET) was used to remove the skull The T 1 scan was used for this, as it has the highest resolution * J. Mazziotta et al. , J. Am. Med. Inform. Assoc. 2001
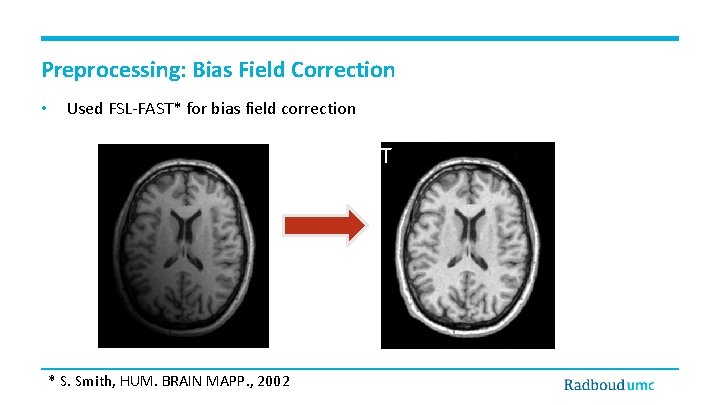
Preprocessing: Bias Field Correction • Used FSL-FAST* for bias field correction FSL FAST * S. Smith, HUM. BRAIN MAPP. , 2002
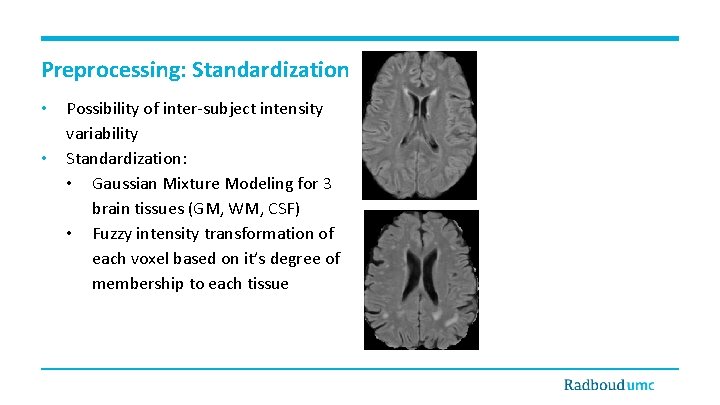
Preprocessing: Standardization • • Possibility of inter-subject intensity variability Standardization: • Gaussian Mixture Modeling for 3 brain tissues (GM, WM, CSF) • Fuzzy intensity transformation of each voxel based on it’s degree of membership to each tissue
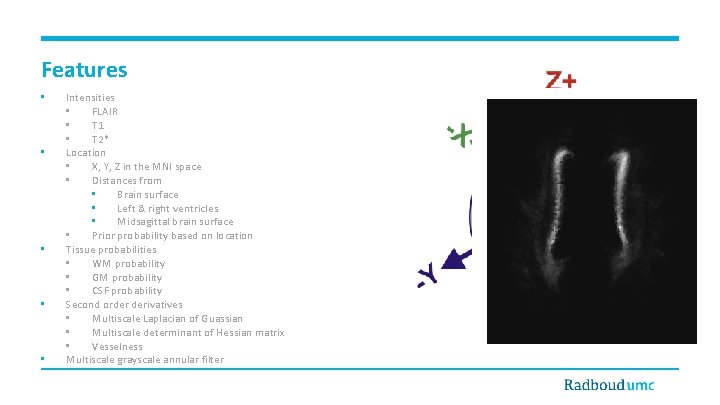
Features • • • Intensities • FLAIR • T 1 • T 2* Location • X, Y, Z in the MNI space • Distances from • Brain surface • Left & right ventricles • Midsagittal brain surface • Prior probability based on location Tissue probabilities • WM probability • GM probability • CSF probability Second order derivatives • Multiscale Laplacian of Guassian • Multiscale determinant of Hessian matrix • Vesselness Multiscale grayscale annular filter
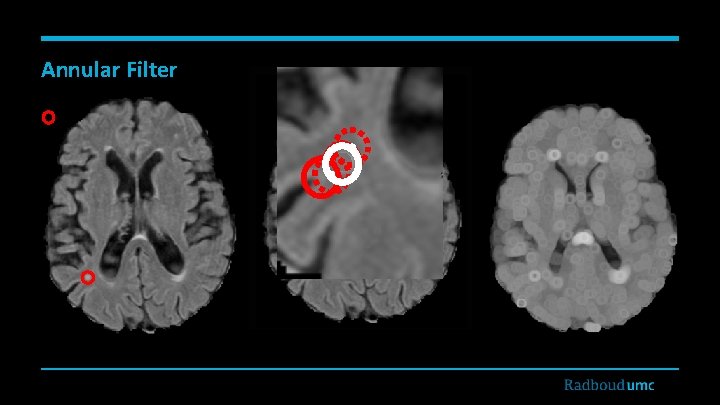
Annular Filter
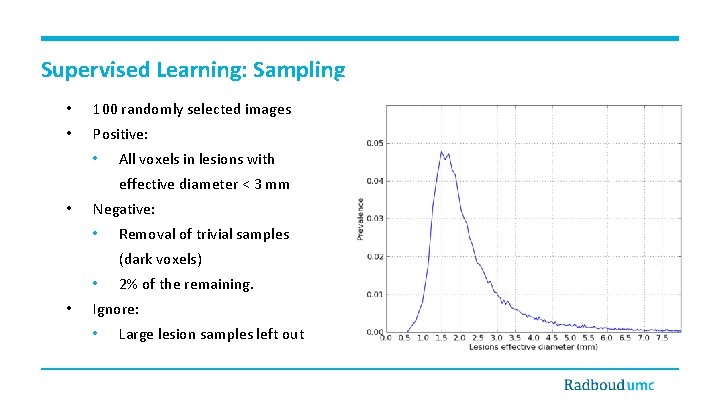
Supervised Learning: Sampling • 100 randomly selected images • Positive: • All voxels in lesions with effective diameter < 3 mm • Negative: • Removal of trivial samples (dark voxels) • • 2% of the remaining. Ignore: • Large lesion samples left out
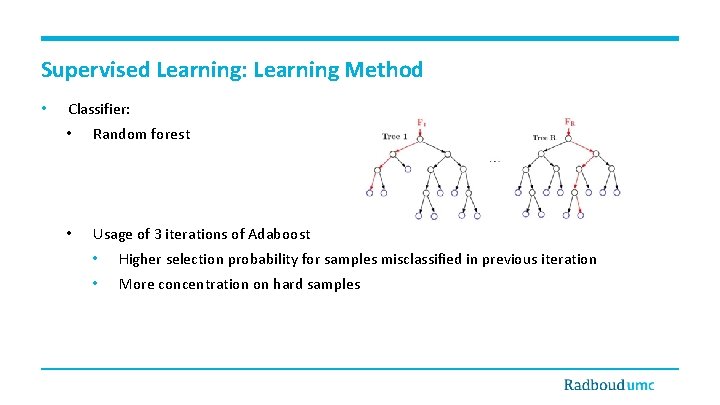
Supervised Learning: Learning Method • Classifier: • Random forest • Usage of 3 iterations of Adaboost • Higher selection probability for samples misclassified in previous iteration • More concentration on hard samples
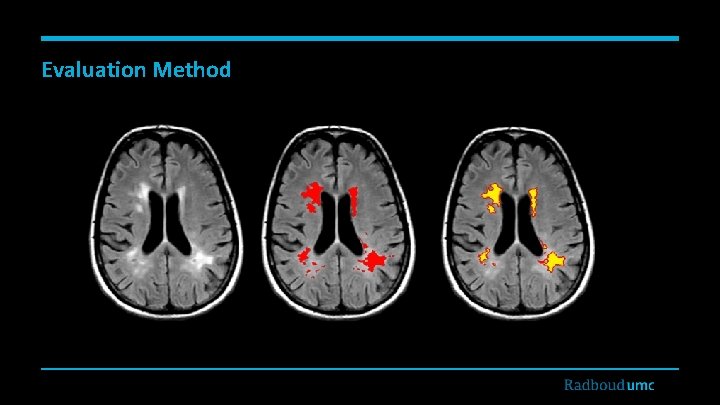
Evaluation Method
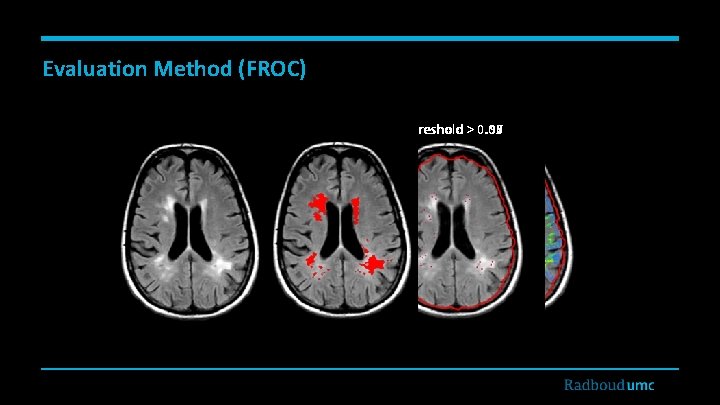
Evaluation Method (FROC) Threshold > 0. 00 0. 99 0. 97 0. 95 FLAIR Annotations Local Maxima Likelihood Map
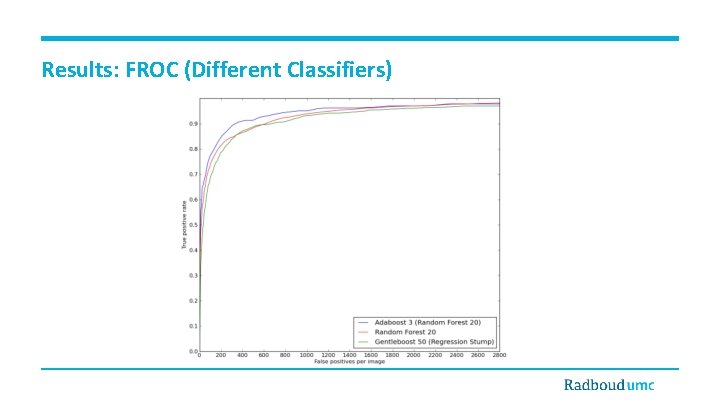
Results: FROC (Different Classifiers)
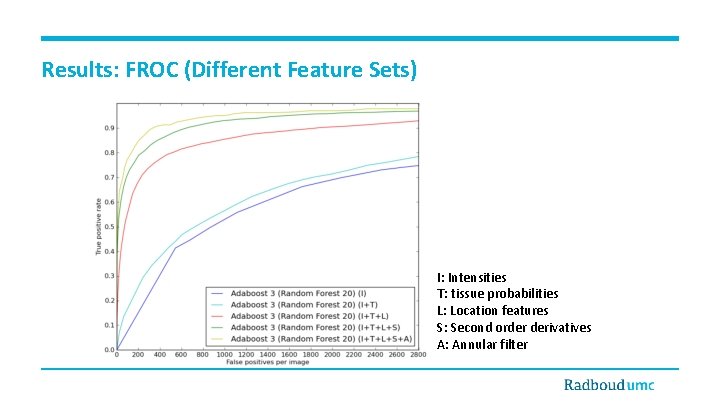
Results: FROC (Different Feature Sets) I: Intensities T: tissue probabilities L: Location features S: Second order derivatives A: Annular filter
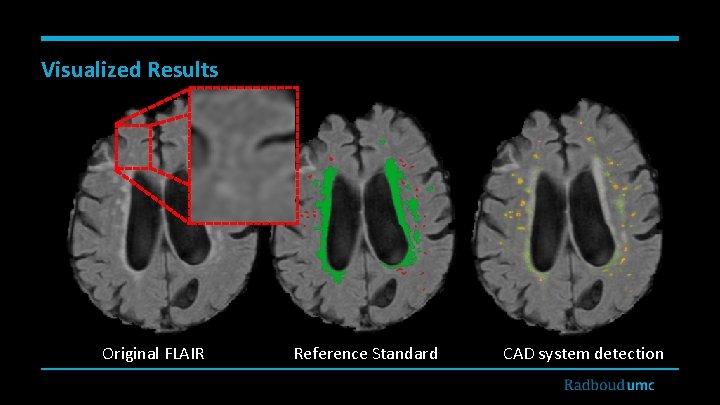
Visualized Results Original FLAIR Reference Standard CAD system detection
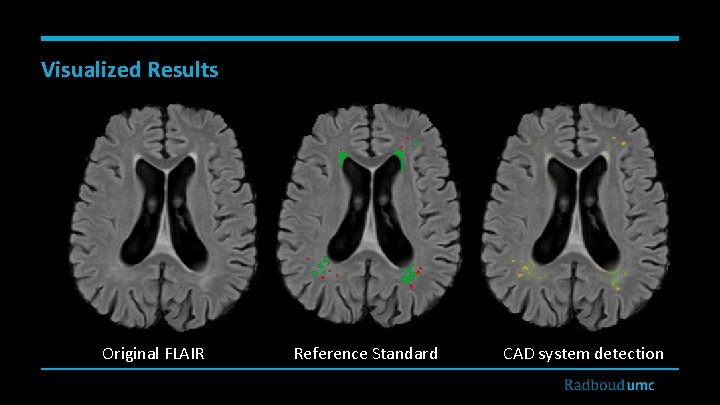
Visualized Results Original FLAIR Reference Standard CAD system detection
Discussion and Conclusions • • • Detection of small WMLs is a challenging task. • Partial volume effect • Dirty white matter • Patient movement artifact and noises Contribution of features • Location information • Annular filter Contribution of classifier • Adaboost

Thanks!
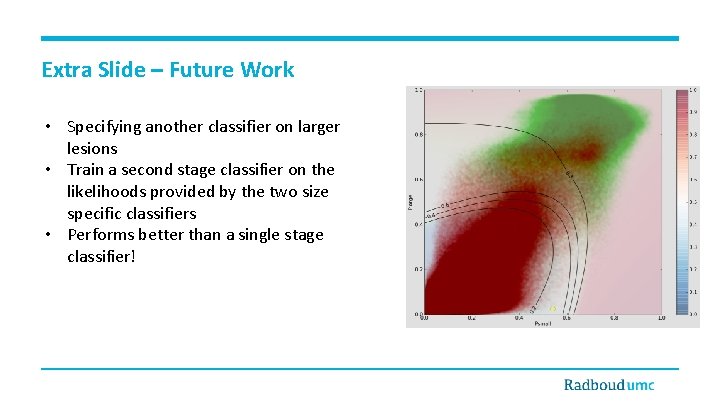
Extra Slide – Future Work • Specifying another classifier on larger lesions • Train a second stage classifier on the likelihoods provided by the two size specific classifiers • Performs better than a single stage classifier!
- Slides: 26