Slides for public AIC CIC Etelcalcetide for treating
![Slides for public [AIC] [CIC] Etelcalcetide for treating of secondary hyperparathyroidism [ID 908] 1 Slides for public [AIC] [CIC] Etelcalcetide for treating of secondary hyperparathyroidism [ID 908] 1](https://slidetodoc.com/presentation_image_h/cb4306718c1d551908ece4412d53de8a/image-1.jpg)
Slides for public [AIC] [CIC] Etelcalcetide for treating of secondary hyperparathyroidism [ID 908] 1 st Committee meeting 8 th February 2017 Committee A Lead team: Justin Daniels, Pam Rees, Ellen Rule ERG: Southampton Health Technology Assessments Centre (SHTAC) NICE technical team: Christian Griffiths, Joanna Richardson, Janet Robertson 1

Key issues for consideration • How are patients treated in clinical practice, is NICE guidance on cinacalcet applied? • Which patients would receive this treatment in clinical practice? • Surrogate biochemical outcomes used in the clinical trials of etelcalcetide. • Is the primary outcome of 30% reduction in PTH level and/or a target of 300 pg/ml (or less) appropriate/generalisable to UK clinical practice? • ERG highlighted that the relative efficacy of etelcalcetide and cinacalcet in patients with refractory SHPT unclear. 2
Secondary hyperparathyroidism (SHPT) • SHPT is a serious and common complication in patients with chronic kidney disease (CKD) on haemodialysis • persistent elevations in levels of biochemical markers of mineral metabolism, including parathyroid hormone (PTH), calcium, and phosphate • if inadequately controlled it is associated with vascular calcification and bone disease (increases risk of cardiovascular events, fractures and death) and reduced quality of life • around 9, 000 of the 21, 000 patients on haemodialysis are estimated to be affected in England • aim of treatment is to maintain parathyroid hormone, calcium and phosphorus levels within acceptable target ranges 3
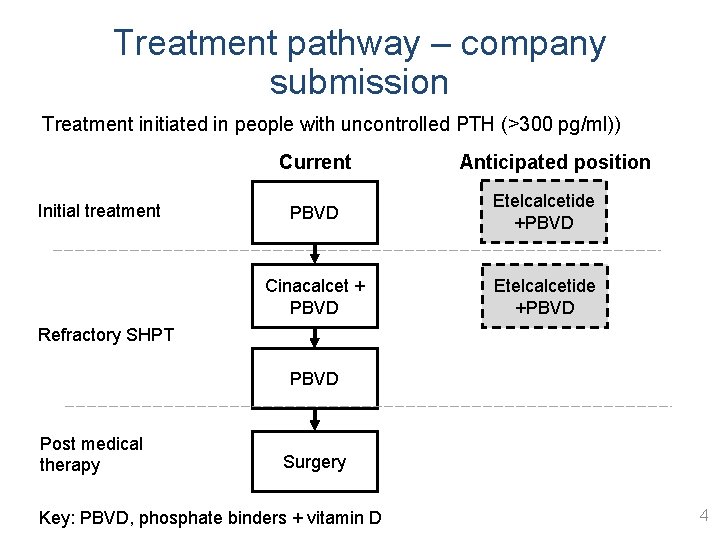
Treatment pathway – company submission Treatment initiated in people with uncontrolled PTH (>300 pg/ml)) Current Initial treatment Anticipated position PBVD Etelcalcetide +PBVD Cinacalcet + PBVD Etelcalcetide +PBVD Refractory SHPT PBVD Post medical therapy Surgery Key: PBVD, phosphate binders + vitamin D 4
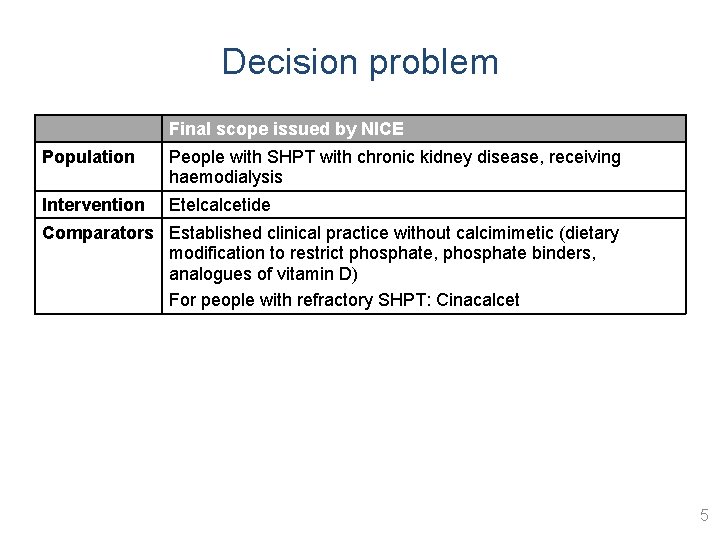
Decision problem Final scope issued by NICE Population People with SHPT with chronic kidney disease, receiving haemodialysis Intervention Etelcalcetide Comparators Established clinical practice without calcimimetic (dietary modification to restrict phosphate, phosphate binders, analogues of vitamin D) For people with refractory SHPT: Cinacalcet 5
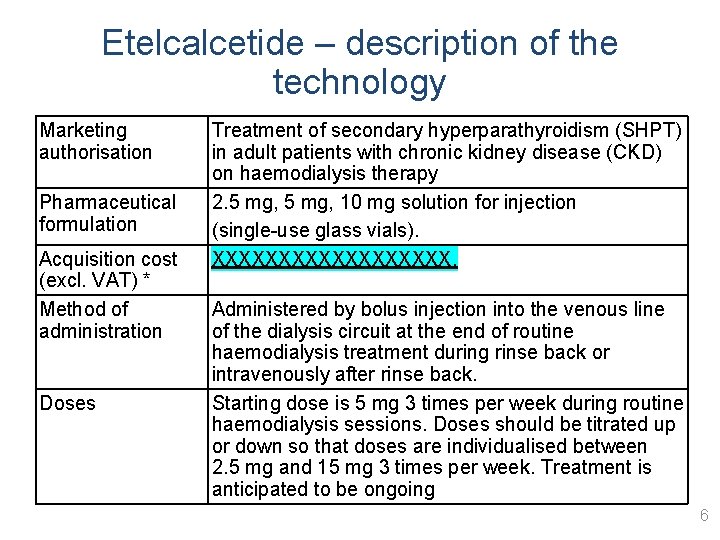
Etelcalcetide – description of the technology Marketing authorisation Pharmaceutical formulation Acquisition cost (excl. VAT) * Method of administration Doses Treatment of secondary hyperparathyroidism (SHPT) in adult patients with chronic kidney disease (CKD) on haemodialysis therapy 2. 5 mg, 10 mg solution for injection (single-use glass vials). XXXXXXXXX. Administered by bolus injection into the venous line of the dialysis circuit at the end of routine haemodialysis treatment during rinse back or intravenously after rinse back. Starting dose is 5 mg 3 times per week during routine haemodialysis sessions. Doses should be titrated up or down so that doses are individualised between 2. 5 mg and 15 mg 3 times per week. Treatment is anticipated to be ongoing 6

Patient perspective (British Kidney Association; Kidney Research UK) • Secondary hyperparathyroidism affects both mental and physical health. • Symptoms include bone pain, stomach pain, fatigue, confusion, nausea and depression leading to mobility problems, sleeplessness and reduced quality of life. • Current NHS treatments do not work for some patients. • Drug regimens are burdensome. Surgery carries extra risk and isn’t always successful. • Patients want relief from symptoms, better control of their condition and for different treatment options to be made available. 7

Patient perspective • Patients and carers have indicated that they expect Etelcalcetide to have the following advantages: • Reduction of pain; Increased mobility; Less need for surgery. • Patients dialysing in hospital do not have the worry of taking another oral medication, as for the first time a calcimimetic will be administered through IV, thus reducing the pill burden. • However, people who are on home dialysis are less likely to want to attend hospital 3 times a week to receive this treatment. 8

Equality Issues • Raised by the British Kidney Association: • “There are kidney patients who are or may be given current treatments off-label, as they are not on dialysis. They may be post-transplant or pre-dialysis and still have secondary PTH and be symptomatic. We would not wish new guidance to impact on this flexibility. There may also be patients with PTH under 800 who benefit from treatment. New treatments should continue for these patients as well. ” 9
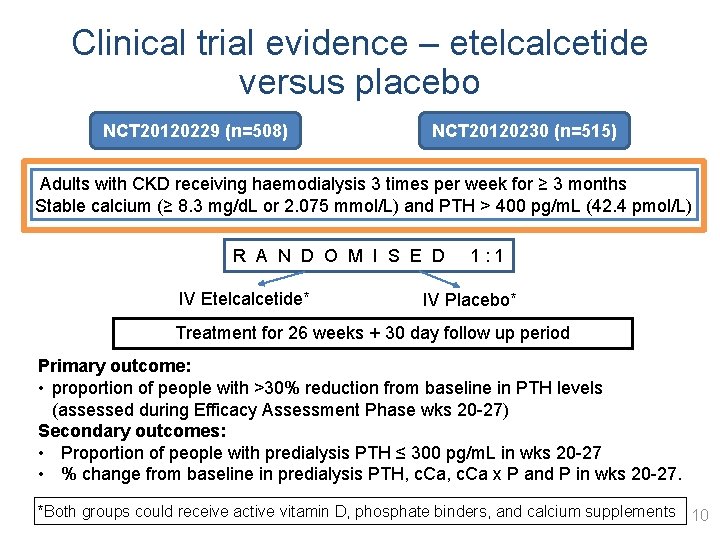
Clinical trial evidence – etelcalcetide versus placebo NCT 20120229 (n=508) NCT 20120230 (n=515) Adults with CKD receiving haemodialysis 3 times per week for ≥ 3 months Stable calcium (≥ 8. 3 mg/d. L or 2. 075 mmol/L) and PTH > 400 pg/m. L (42. 4 pmol/L) R A N D O M I S E D 1 : 1 IV Etelcalcetide* IV Placebo* Treatment for 26 weeks + 30 day follow up period Primary outcome: • proportion of people with >30% reduction from baseline in PTH levels (assessed during Efficacy Assessment Phase wks 20 -27) Secondary outcomes: • Proportion of people with predialysis PTH ≤ 300 pg/m. L in wks 20 -27 • % change from baseline in predialysis PTH, c. Ca x P and P in wks 20 -27. *Both groups could receive active vitamin D, phosphate binders, and calcium supplements 10
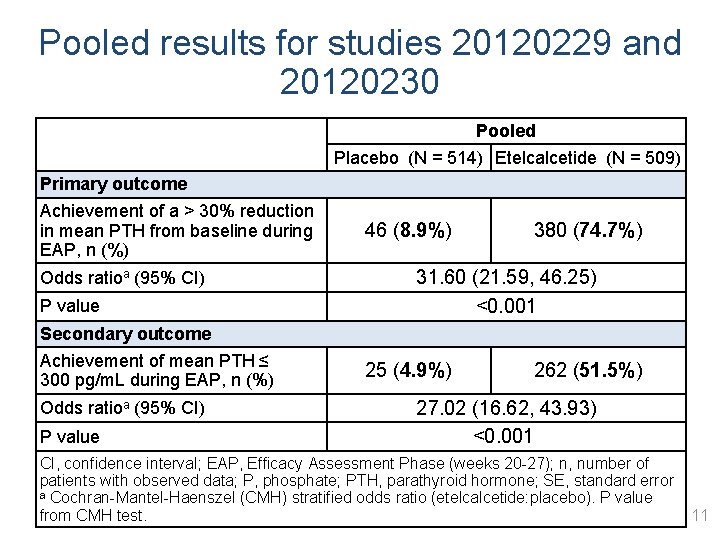
Pooled results for studies 20120229 and 20120230 Pooled Placebo (N = 514) Etelcalcetide (N = 509) Primary outcome Achievement of a > 30% reduction in mean PTH from baseline during EAP, n (%) Odds ratioa (95% CI) P value Secondary outcome Achievement of mean PTH ≤ 300 pg/m. L during EAP, n (%) Odds ratioa (95% CI) P value 46 (8. 9%) 380 (74. 7%) 31. 60 (21. 59, 46. 25) <0. 001 25 (4. 9%) 262 (51. 5%) 27. 02 (16. 62, 43. 93) <0. 001 CI, confidence interval; EAP, Efficacy Assessment Phase (weeks 20 -27); n, number of patients with observed data; P, phosphate; PTH, parathyroid hormone; SE, standard error a Cochran-Mantel-Haenszel (CMH) stratified odds ratio (etelcalcetide: placebo). P value from CMH test. 11
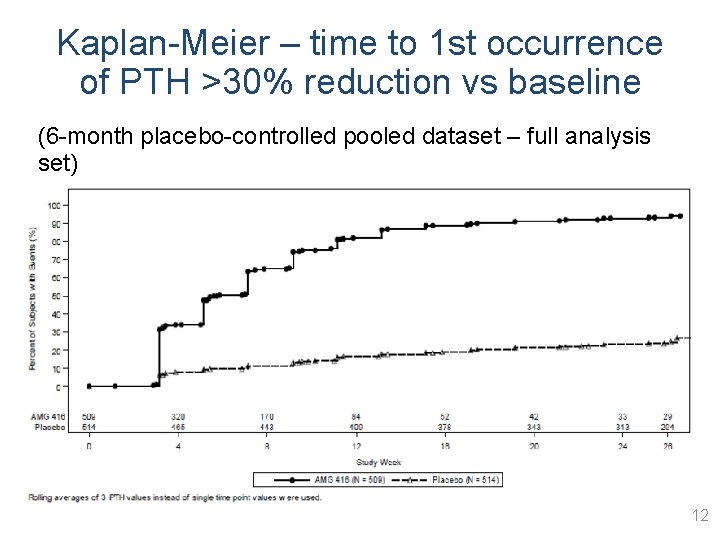
Kaplan-Meier – time to 1 st occurrence of PTH >30% reduction vs baseline (6 -month placebo-controlled pooled dataset – full analysis set) 12
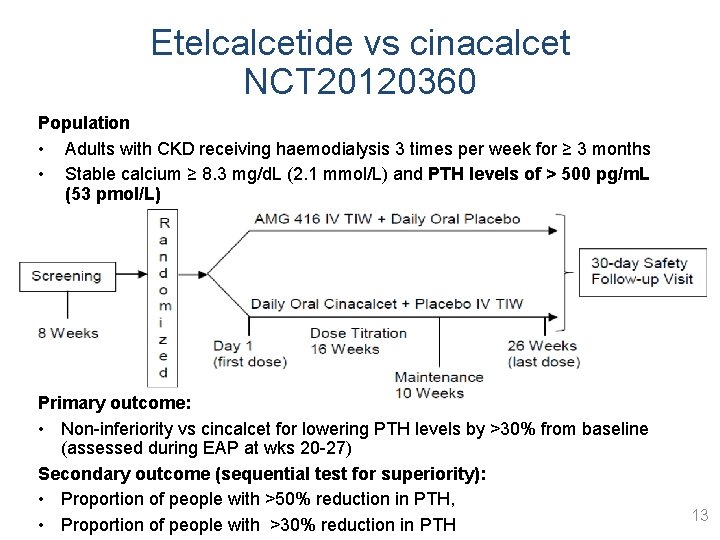
Etelcalcetide vs cinacalcet NCT 20120360 Population • Adults with CKD receiving haemodialysis 3 times per week for ≥ 3 months • Stable calcium ≥ 8. 3 mg/d. L (2. 1 mmol/L) and PTH levels of > 500 pg/m. L (53 pmol/L) Primary outcome: • Non-inferiority vs cincalcet for lowering PTH levels by >30% from baseline (assessed during EAP at wks 20 -27) Secondary outcome (sequential test for superiority): • Proportion of people with >50% reduction in PTH, • Proportion of people with >30% reduction in PTH 13
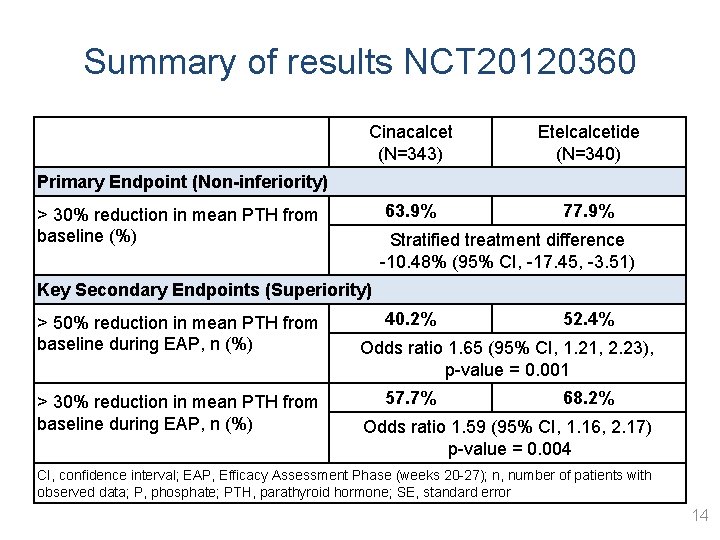
Summary of results NCT 20120360 Cinacalcet (N=343) Etelcalcetide (N=340) 63. 9% 77. 9% Primary Endpoint (Non-inferiority) > 30% reduction in mean PTH from baseline (%) Stratified treatment difference -10. 48% (95% CI, -17. 45, -3. 51) Key Secondary Endpoints (Superiority) > 50% reduction in mean PTH from baseline during EAP, n (%) > 30% reduction in mean PTH from baseline during EAP, n (%) 40. 2% 52. 4% Odds ratio 1. 65 (95% CI, 1. 21, 2. 23), p-value = 0. 001 57. 7% 68. 2% Odds ratio 1. 59 (95% CI, 1. 16, 2. 17) p-value = 0. 004 CI, confidence interval; EAP, Efficacy Assessment Phase (weeks 20 -27); n, number of patients with observed data; P, phosphate; PTH, parathyroid hormone; SE, standard error 14
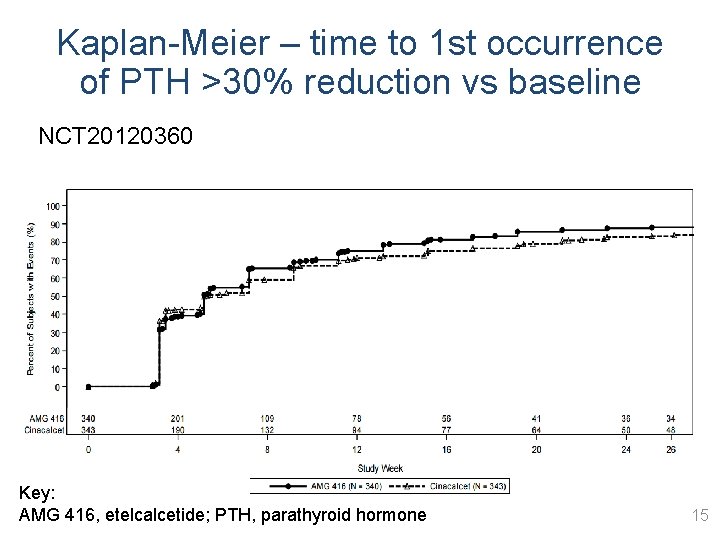
Kaplan-Meier – time to 1 st occurrence of PTH >30% reduction vs baseline NCT 20120360 Key: AMG 416, etelcalcetide; PTH, parathyroid hormone 15
Subgroups • Pre-specified subgroup analyses • Studies 20120229, 20120230 and 20120360 – Based on demographics, severity of SHPT and prior use of cinacalcet – Company state that superior efficacy of etelcalcetide over the comparators was consistent across all pre-defined patient subgroups • ERG comments – Agree that there were no significant differences in efficacy between the whole trial populations and the pre-specified subgroups – Caution required as the subgroup analyses were not statistically powered to detect treatment differences 16
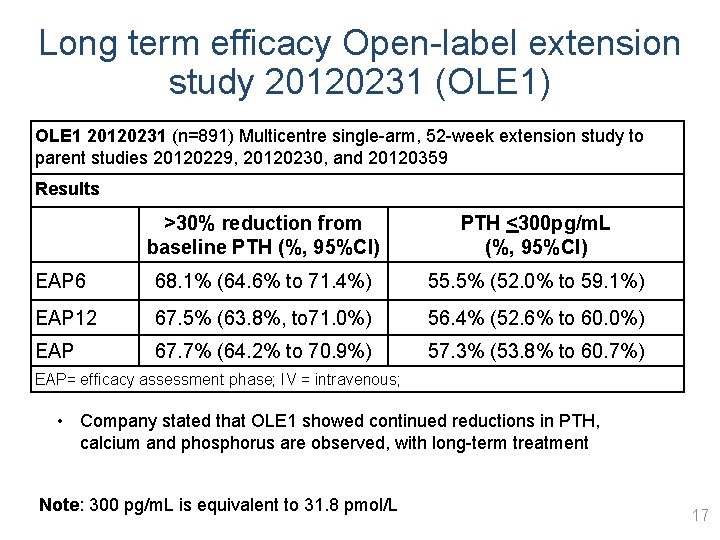
Long term efficacy Open-label extension study 20120231 (OLE 1) OLE 1 20120231 (n=891) Multicentre single-arm, 52 -week extension study to parent studies 20120229, 20120230, and 20120359 Results >30% reduction from baseline PTH (%, 95%CI) PTH <300 pg/m. L (%, 95%CI) EAP 6 68. 1% (64. 6% to 71. 4%) 55. 5% (52. 0% to 59. 1%) EAP 12 67. 5% (63. 8%, to 71. 0%) 56. 4% (52. 6% to 60. 0%) EAP 67. 7% (64. 2% to 70. 9%) 57. 3% (53. 8% to 60. 7%) EAP= efficacy assessment phase; IV = intravenous; • Company stated that OLE 1 showed continued reductions in PTH, calcium and phosphorus are observed, with long-term treatment Note: 300 pg/m. L is equivalent to 31. 8 pmol/L 17

Health-related quality of life • Collected as part of study 20120360 (etelcalcetide vs cinacalcet) • Measured using KDQOL-36 (has 5 sub-scales …. • XXXXXXXXXXXXXXXXXXXXXXX – XXXXXXXXXXXXXXXXXXX performed) – XXXXXXXXXXXXXXXXXXXX • The company did not use these results in the economic base case analysis and no HRQo. L benefit is assumed for calcimimetic treatment in the base case (although a scenario analyses explored this) • ERG agrees that XXXXXXXX HRQo. L scores from the KDQOL-36 results, the company’s approach was reasonable 18
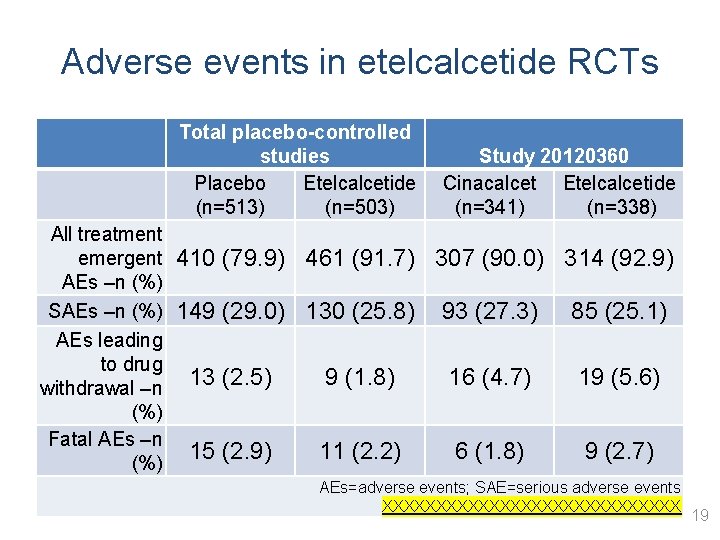
Adverse events in etelcalcetide RCTs • fdfd Total placebo-controlled studies Placebo Etelcalcetide (n=513) (n=503) All treatment emergent 410 (79. 9) 461 (91. 7) AEs –n (%) SAEs –n (%) 149 (29. 0) 130 (25. 8) AEs leading to drug 13 (2. 5) 9 (1. 8) withdrawal –n (%) Fatal AEs –n 15 (2. 9) 11 (2. 2) (%) Study 20120360 Cinacalcet Etelcalcetide (n=341) (n=338) 307 (90. 0) 314 (92. 9) 93 (27. 3) 85 (25. 1) 16 (4. 7) 19 (5. 6) 6 (1. 8) 9 (2. 7) AEs=adverse events; SAE=serious adverse events XXXXXXXXXXXXXX 19
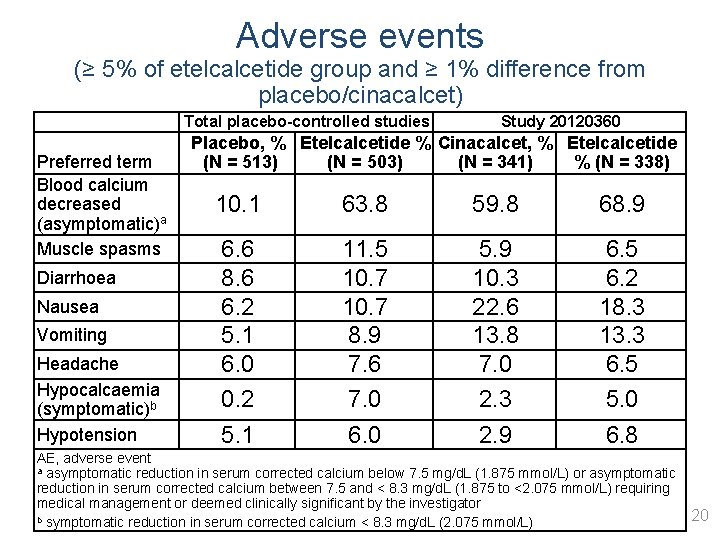
Adverse events (≥ 5% of etelcalcetide group and ≥ 1% difference from placebo/cinacalcet) Preferred term Blood calcium decreased (asymptomatic)a Muscle spasms Diarrhoea Nausea Vomiting Headache Hypocalcaemia (symptomatic)b Hypotension Total placebo-controlled studies Study 20120360 Placebo, % Etelcalcetide % Cinacalcet, % Etelcalcetide (N = 513) (N = 503) (N = 341) % (N = 338) 10. 1 63. 8 59. 8 68. 9 6. 6 8. 6 6. 2 5. 1 6. 0 0. 2 5. 1 11. 5 10. 7 8. 9 7. 6 7. 0 6. 0 5. 9 10. 3 22. 6 13. 8 7. 0 2. 3 2. 9 6. 5 6. 2 18. 3 13. 3 6. 5 5. 0 6. 8 AE, adverse event a asymptomatic reduction in serum corrected calcium below 7. 5 mg/d. L (1. 875 mmol/L) or asymptomatic reduction in serum corrected calcium between 7. 5 and < 8. 3 mg/d. L (1. 875 to <2. 075 mmol/L) requiring medical management or deemed clinically significant by the investigator 20 b symptomatic reduction in serum corrected calcium < 8. 3 mg/d. L (2. 075 mmol/L)

ERG comments clinical effectiveness (1) • Good quality trials although unclear if double-blinding was preserved, some results not ITT (risk of attrition bias) • People included in trials were generally representative of those seen in practice in the UK • Submission may not provide evidence about the efficacy of etelcalcetide vs cinacalcet in refractory SHPT population – Trial included broad population of patients with SHPT, rather than those with refractory SHPT • Trials did not measure the longer-term clinically relevant outcomes specified in the scope 21

ERG comments clinical effectiveness (2) • Drug doses in the trials titrated to a PTH target of <300 pg/m. L (31. 8 pmol/L) – ERG suggest that in practice 130 – 600 pg/m. L (13. 8 – 63. 6 pmol/L) would be acceptable depending on Ca and P parameters • Target used in trials did not include a lower range cut-off, therefore some at risk of PTH over-suppression (could impact longer term outcomes and cost effectiveness) 22

Key issues for consideration • How are patients treated in clinical practice, is NICE guidance on cinacalcet applied? • Which patients would receive this treatment in clinical practice? • Surrogate biochemical outcomes used in the clinical trials of etelcalcetide. • Is the primary outcome of 30% reduction in PTH level and/or a target of 300 pg/ml (or less) appropriate/generalisable to UK clinical practice? • ERG highlighted that the relative efficacy of etelcalcetide and cinacalcet in patients with refractory SHPT unclear. 23
- Slides: 23